Abstract
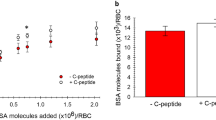
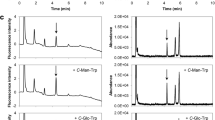
An affinity purification procedure is employed for the isolation of FL-specific binding proteins from MM6 cell membranes using magnetobeads coated with glycated polylysine and elution with FL and glycated 6-aminocaproic acid. Two main binding proteins were identified as membrane-bound nucleolin and cellular myosin heavy chain, which are glycosylated. This study shows that in these cells binding of short-term glycated albumin leads to activation of PKC, especially its isoform ε and this is linked to translocation of AP-1 and NF-κB into the nucleus. Consequently, an increased formation of IL-1ß mRNA is observed. The PKC inhibitor GÖ6976 prevents all these effects. Glycated albumin also stimulates activation of PTK. The PTK inhibitor genistein prevents activation of AP-1 indicating that PTK is also involved in this process, whereas NF-κB translocation is only dependent on PKC activation.
Similar content being viewed by others
References
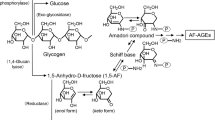
Krantz S, Lober M, Henschel L, The nonenzymatic glycation of proteins and nucleic acids, their importance for the development of diabetic complications, possible molecular basis of aging and autoimmunological processes, Exp Clin Endocrinol 88, 257–69 (1986).
Krantz S, Brandt R, Gromoll B, Binding sites for short-term glycated albumin on peritoneal cells of the rat, Biochim Biophys Acta 1177, 15–24 (1993).
Salazar R, Brandt R, Krantz S, Expression of fructosyllysine receptors on human monocytes and monocyte-like cell lines, Biochim Biophys Acta 1266, 57–63 (1995).
Brandt R, Landmesser C, Vogt L, Hehmke B, Hanschke R, Kasbohm J, Hartmann K, Jäger B, Krantz S, Michaelis D, Differential expression of fructosyllysine-specific receptors on monocytes and macrophages and possible pathophysiological significance, Diabetologia 39, 1140–7 (1996).
Wu V-Y, Cohen MP, Identification of aortic endothelial cell binding proteins for Amadori adducts in glycated albumin, Biochem Biophys Res Commun 193, 1131–6 (1993).
Wu V-Y, Cohen MP, Receptor specific for Amadori-modified glycated albumin on murine endothelial cells, Biochem Biophys Res Commun 198, 734–9 (1994).
Wu V-Y, Cohen MP, Evidence for a ligand receptor system mediating the biological effects of glycated albumin in glomerular mesangial cells, Biochem Biophys Res Commun 207, 521–8 (1995).
Cohen MP, Hud E, Wu V-Y, Ziyadeh FN, Albumin modified by Amadori glucose adduct activates mesangial cell type IV collagen gene transcription, Mol Cell Biochem 143, 73–9 (1995).
Cohen MP, Ziyadeh FN, Role of Amadori-modified nonenzymatically glycated serum proteins in the pathogenesis of diabetic nephropathy, J Am Soc Nephrol 7, 183–90 (1996).
Finot PA, Mauron J, Le blockage de la lysine par le reaction de Maillard: Synthese de N-(deoxy-1-D-fructosyl-1)-et N-(deoxy-1-D-lactulosyl-1)-L-lysine, Helv Chim Acta 52, 1488–95 (1969).
Salazar R, Brandt R, Kellermann J, Krantz S, Purification and characterization of a 200 kDa fructosyllysine-specific binding protein from cell membranes of U937 cells, Glycoconjugate J 17, 713–6 (2000).
Bierhaus A, Chevion S, Chevion M, Hofmann M, Quehenberger P, Illner T, Luther T, Berenshtein E, Tritschler H, Müller M, Wahl P, Ziegler R, Nawroth PP, Advanced glycation endproduct-induced activation of NF-κB is suppressed by α-lipoic acid in cultured endothelial cells, Diabetes 46, 1481–90 (1997).
Brownlee M, Advanced protein glycosylation in diabetes and aging, Annu Rev Med 46, 223–34 (1995).
Lyons TJ, Glycation, oxidation, and glycoxidation reactions in the development of diabetic complications, Contributions to Nephrology 112, 1–10 (1995).
Vlassara H, Bucala R, Advanced glycation and diabetes complication: An update, Diabetes Annual 9, 227–44 (1995).
Verbeke P, Perichon M, Schaeverbeke J, Bakala H, Effect of glycation of albumin on its binding to renal brush-border membrane vesicles; influence of aging in rats, Biochim Biophys Acta 1282, 93–100 (1996).
Krantz S, Salazar R, Brandt R, Kellermann J, Lottspeich F, Purification and partial amino acid sequencing of fructosyllysinespecific binding protein from cell membranes of the monocyte-like cell line U937, Biochim Biophys Acta 1266, 109–12 (1995).
Srivastava M, Pollard HB, Molecular dissection of nucleolin's role in growth and cell proliferation: New insights, FASEB J 13, 1911–22 (1999).
Ginisty H, Sicard H, Roger B, Bouvet P, Structure and functions of nucleolin, J Cell Sci 11, 761–72 (1999).
Semenkovich CF, Ostlund RE, Olson MDJ, Yang JW, A protein partially expressed on the surface of HpG2 cells that binds lipoproteins specifically is nucleolin, Biochemistry 29, 9708–13 (1990).
Kleinman HK, Weeks BS, Cannon TB, Sweeney TM, Sephel GC, Clement B, Zain M, Olson MDJ, Jucker N, Burrous BA, Identification of a 110-kDa nonintegrin cell surface laminin-binding protein which recognizes an A chain neurite-promoting peptide, Arch Biochem Biophys 290, 320–5 (1991).
Qiu J, Brown KE, A 110-kDa nuclear shuttle protein, nucleolin, specifically binds to adeno-associated virus type 2 (AAV-2) capsid, Virology 257, 373–82 (1999).
Toothaker LE, Gonzales DA, Tung N, Lemons RS, LeBeau MM, Arnaout MA, Clayton LK, Tenen DG, Cellular myosin heavy chain in human leukocytes: Isolation of 5′ cDNAclones, characterization of the protein, chromosomal localization, and upregulation during myeloid differentiation, Blood 78, 1826–33 (1991).
Li D, Miller M, Chantler PD, Association of a cellular myosin II with anionic phospholipids and the neuronal plasma membrane, Proc Natl Acad Sci USA 91, 853–7 (1994).
Srivastava M, Fleming PJ, Pollard MB, Burns AL, Cloning and sequencing of human nucleolin cDNA, FEBS-Lett 250, 99–105.
Watkins LR, Hansen MK, Nguyen KT, Lee JE, Maier SV, Dynamic regulation of the proinflammatory cytokine, interleukin 1beta: Molecular biology for non-molecular biologists, Life Sci 65, 449–81 (1999).
Kim SJ, Denhez F, Kim KY, Holt JT, Sporn MB, Roberts AB, Activation of the second promoter of the transforming growth factor beta1 gene by transforming growth factor beta1 and phorbol ester occurs through the same target sequences, J Biol Chem 264, 193973–8 (1989).
Schultze S, Machleidt T, Kronke M, Mechanisms of tumor necrosis factor action, Seminars in Oncology 19(Suppl 4), 16–24 (1992).
Lu Y, Granelli-Piperno A, Bjorndahl JM, Phillips CA, Trevillyan JM, CD28 induced T cell activation. Evidence for a proteintyrosine kinase signal transduction pathway, J Immunol 149, 24–9 (1992).
Ueffing M, Lovric J, Philipp A, Mischak H, Kolch W, Protein kinase C-ε associates with the Raf-1 kinase and induces the production of growth factors that stimulate Raf-1 activity, Oncogene 15, 2921–7 (1997).
Steffan NM, Bren GD, Frantz B, Tocci MJ, O'Neill EA, Paya CV, Regulation of IκBalpha phosphorylation by pKC-and Ca2+-dependent signal transduction pathways, J Immunol 155, 4685–91 (1995).
Chen S, Cohen MP, Ziyadeh FN, Amadori-glycated albumin in diabetic nephropathy: Pathophysiologic connections, Kidney Int 58(Suppl 77), S-40–S-44 (2000).
Cohen MP, Wu V-Y, Cohen JA, Glycated albumin stimulates fibronectin and collagen IV production by glomerular endothelial cells under normoglycemic conditions, Biochem Biophys Res Commun 239, 91–4 (1997).
Chen S, Cohen MP, Lautenslager G, Shearman CW, Ziyadeh FN, Glycated albumin stimulates TGF-ß1 production and protein kinase C activity in glomerular endothelial cells, Kidney Int 59, 673–81 (2001).
Cohen MP, Ziyadeh FN, Lautenslager GT, Cohen JA, Shearman CW, Glycated albumin stimulation of PKC-ß activity is linked to increased collagen IV in mesangial cells, Am J Physiol 276, F684–90 (1999).
Ziyadeh FN, Renal tubular basement membrane and collagen type IV in diabetes mellitus, Kidney Int 43, 114–20 (1993).
Mandl-Weber S, Haslinger B, Schalkwijk CG, Sitter T, Early glycated albumin, but not advanced glycated albumin, methylglyoxal, or 3-deoxyglucosone increases the expression of PAI-I in human peritoneal mesothelial cells, Perit Dial Int 21, 487–94 (2001).
Hattori Y, Suzuki M, Hattori S, Kasai K, Vascular smooth muscle cell activation by glycated albumin (Amadori adducts), Hypertension 39, 22–8 (2002).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Salazar, R., Brandt, R. & Krantz, S. Binding of Amadori glucose-modified albumin by the monocytic cell line MonoMac 6 activates protein kinase Cε protein tyrosine kinases and the transcription factors AP-1 and NF-κB. Glycoconj J 18, 769–777 (2001). https://doi.org/10.1023/A:1021151417556
Issue Date:
DOI: https://doi.org/10.1023/A:1021151417556