Abstract
Conditional stability constants of 2-[bis(2-hydroxyethyl)amino]-2(hydroxymethyl)-1,3-propanediol (BT) complexes of trivalent rare earth element (Ln) ions (La, Nd, Eu, Gd, Yb, Dy, Er, Lu) and Y were determined potentiometrically in aqueous NaCl solutions at 30°C and 0.1 M ionic strength. Least-squares fitting shows that, at <0.04 molal BT, the complex LnBT3+ is dominant, with LnBT2 3+ forming a secondary complex, where:
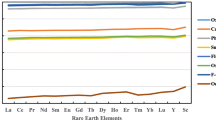
Conditional stability constants appear to be directly related to the ionic radius of the trivalent ion in question. The optimal ionic radius, 104–105 pm, yields values of log \(\beta _{{21}}^{*} = 10.93 \pm 0.63\) (Gd) and \(\beta _{{11}}^{*} = 6.83 \pm 0.14\) (Yb). Complexation drops off steeply on either side of the ideal ionic radius. In relating the stability constants to ionic radius, it is assumed that BT complexes with Gd, Dy, Er, and Lu have coordination number eight, whereas those with La, Nd, and Eu have coordination number nine. The smoothest trend of stability constants with ionic radius is obtained if Yb–BT complexes are assumed to have coordination number nine. These results may reflect the ability of BT to form an ionic radius-specific chelate structure.
Similar content being viewed by others
References
A. L. Olson, 25th Actinide Separations Conf. p. 8(2001).
J. C. Lewis, Anal. Biochem. 14, 495(1966).
K. H. Scheller, T. H. J. Abel, P. E. Polanyi, P. K. Wenk, B. E. Fischer, and H. Sigel, Eur. J. Biochem. 107, 455(1980).
H. Sigel, K. H. Scheller and B. Prijs, Inorg. Chem. Acta. 6, 147(1982).
J. M. Pfefferié and J. C. Bünzli, Helv. Chimi. Acta. 72, 1487(1989).
D. J. Wesolowski, D. A. Palmer, and G. M. Begun, J. Solution Chem. 19, 159(1990).
K. H. Hong, E. J. Ha, and K. S. Bai, Bull. Korean Chem. Soc. 16, 406(1995).
Q. Chen, Y. D. Chang, and J. Zubieta, Inorg. Chim. Acta 258, 257(1997).
S. J. Oh, Y. S. Choi, S. Hwangbo, S. C. Bae, J. K. Ku, and J. W. Park, Chem. Commun, p. 2189(1998).
P. Gómez-Tagle and A. K. Yatsimirsky, Inorg. Chem. 40, 3786(1998).
J. A. Winchester and P. A. Floyd, Chem. Geol. 20, 325(1977).
D. A. Wood, J. Tarney, and B. L. Weaver, Tectonophysics 75, 91(1981).
J. W. Shervais, Earth Plant. Sci. Let. 51, 101(1982).
J. S. Seewald and W. E. Seyfried, Jr., Geochim. Cosmochim. Acta 55, 659(1990).
P. L. Hellman and P. Henderson, Nature 267, 38(1977).
P. L. Hellman, R. E. Smith, and P. Henderson, Cont. Min. Pet. 71, 23(1979).
B. Bock, S. M. McLennan, and G.N. Hanson, Geochim. Cosmochim. Acta 58, 5245(1994).
D. K. McDaniel, S. R. Hemming, S. M. McLennan, and G.N. Hanson, Geochim. Cosmochim. Acta 58, 931(1994).
M. Ohr, A. N. Halliday, and D. R. Peacor, Geochim. Cosmochim. Acta 58, 289(1994).
D. J. Kontak and S. Jackson, Can. Min. 33, 445(1995).
I. M. Kolthoff, E. B. Sandell, E. J. Meehan, and S. Bruckenstein, Quantitative Chemical Analyses (Macmillian, NY, 1969).
R. Ding and S. A. Wood, Geochem. Soc. Special Publ. 7, 209(2002
F. H. Spedding, M. J. Pikal, and B. O. Ayers, J. Phys. Chem. 70, 2440(1966).
F. H. Spedding, P. F. Cullen, and A. Habenschuss, J. Phys. Chem. 78, 1106(1974).
F. H. Spedding, J. A. Rard, and A. Habenschuss, J. Phys. Chem. 81, 1069(1977).
J. C. G. Bünzli and G. R. Choppin, Lanthanide Probes in Life, Chemical and Earth Sciences. Theory and Practice (Elsevier, New York, 1989).
S. A. Wood, Chem Geol. 82, 159(1990).
C. F. Baes and R. E. Mesmer, The Hydrolysis of Cations (Wiley, NY, 1976).
A. E. Martell and R. J. Motekaitis, Determination of Stability Constants, 2nd edn. (VCH Publishers, NY, 1992).
C. H. Gammons, S. A. Wood, and A. E. Williams-Jones, Geochim. Cosmochim, Acta 60, 4615(1996).
F. J. Millero, Geochim. Cosmochim. Acta 56, 3123(1992).
B. M. Fabuss, A. Korosi, and A. K. M. Shamsul Huq, J.Chem. Eng. Data 11, 325(1966).
R. H. Busey and R. E. Mesmer, J.Chem. Eng. Data 23, 175(1978).
M. Paabo and G. J. Bates, J. Phys. Chem. 65, 667(1970).
C. D. McGlothlin and J. Jordan, Anal. Lett. 9, 245(1976).
Y. Kitamura and T. Itoh, J. Solution Chem. 16, 715(1987).
D. J. Wesolowski and D. A. Palmer, J. Solution Chem. 18, 545(1989).
S. A. Wood, D. A. Palmer, D. J. Wesolowski, and P. Bénézeth, Geochem. Soc. Special Publ. 7, 229(2002
S. Aime, M. Botta, M. Fasano, M. Paula, M. Marques, C. F. G. C. Geraldes, D. Pubanz, and A. E. Merbach, Inorg. Chem. 36, 2059(1997).
Greenwood and Earnshaw, Chemistry of the Elements (Butterworth-Heinemann, Oxford, 1989).
F. A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann, Advanced Inorganic Chemistry, 6th edn. (Wiley, NY, 1999).
R. D. Shannon, Acta Crystallogr. A. 32, 751(1976).
K. N. Nicholson, B. Twamley, and S. A. Wood, Acta Crystallogr. 57, 1133(2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nicholson, K.N., Wood, S.A. Aqueous Geochemistry of Rare Earth Elements and Yttrium. XII: Potentiometric Stability Constant Determination of Bis-Tris Complexes with La, Nd, Eu, Gd, Yb, Dy, Er, Lu, and Y. Journal of Solution Chemistry 31, 703–717 (2002). https://doi.org/10.1023/A:1021128907144
Issue Date:
DOI: https://doi.org/10.1023/A:1021128907144