Abstract
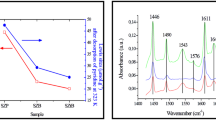
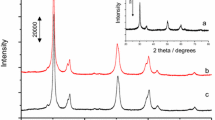
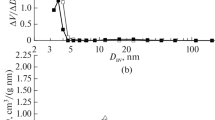
The benzoylation of anisole with benzoic anhydride over sulfated zirconia has been investigated, especially with regard to catalyst deactivation and regeneration. Calcined sulfated zirconia and spent catalysts have been characterized by BET analysis, C-H elemental analysis, DRIFTS, NH3-TPD, 13C-NMR and UV-Vis spectroscopy. In batch-mode experiments, a moderate deactivation attributed to the deposition of carbonaceous surface species has been observed. The surface deposits consist of benzoate species and strongly adsorbed molecules of anisole. A color change of the solid, observed immediately after the introduction of the catalyst into the reaction mixture, has been attributed to the protonation of the reaction products 2- and/or 4-methoxybenzophenone. The surface species formed may cover or modify surface acid sites and, in this way, reduce the accessibility of active centers. Removing the deposited and adsorbed species by calcination in air restores the initial activity of sulfated zirconia.
Similar content being viewed by others
References
A. Corma, Chem. Rev. 95 (1995) 559.
B.M. Choudary, M. Sateesh, M.L. Kantam and K.V.R. Prasad, Appl. Catal. A 171 (1998) 155.
C. deCastro, J. Primo and A. Corma, J. Mol. Catal. A 134 (1998) 215.
M. Spangol, L. Gilbert, E. Benazzi and C. Marcilly, PCT Int. Appl. WO 9635, 655 (1996).
K. Tanabe, T. Yamaguchi, K. Akiyama, A. Mitoh, K. Iwabuchi and K. Isogai, Proc. 8th Intern. Congr. Catal. (Verlag Chemie, Weinheim, 1984), Vol. 5, p. 601.
T. Yamaguchi, T. Jin, T. Ishida and K. Tanabe, Mater. Chem. Phys. 17 (1987) 3.
V. Quaschning, J. Deutsch, P. Druska, H.J. Niclas and E. Kemnitz, J. Catal. 177 (1998) 164.
G.D. Yadav and A.A. Pujari, Green Chem. 1 (1999) 69.
J. Deutsch, V. Quaschning, E. Kemnitz, A. Auroux, H. Ehwald and H. Lieske, Topics Catal. 13 (2000) 281.
V. Quaschning, A. Auroux, J. Deutsch, H. Lieske and E. Kemnitz, J. Catal. 203 (2001) 426.
D. Rohan, C. Canaff, E. Fromentin and M. Guisnet, J. Catal. 177 (1998) 296.
E.G. Derouane, C.J. Dillon, D. Bethell and S.B. Derouane-Abd Hamid, J. Catal. 187 (1999) 209.
U. Freese, F. Heinrich and F. Roessner, Catal. Today 49 (1999) 237.
P. Moreau, A. Finiels and P. Meric, J. Mol. Catal. A 154 (2000) 185.
E.G. Derouane, G. Crehan, C.J. Dillon, D. Bethell, H. He and S.B. Derouane-Abd Hamid, J. Catal. 194 (2000) 410.
P. Laidlaw, D. Bethell, S.M. Brown and G.J. Hutchings, J. Mol. Catal. A 74 (2001) 187.
L. Cerveny, K. Mikulcova and J. Cejka, Appl. Catal. A 223 (2002) 65.
H.T. Clarke and E.J. Rahrs, Org. Synth., Coll. Vol. I, 91 (1941).
T. Jin, T. Yamaguchi and K. Tanabe, J. Phys. Chem. 90 (1986) 4794.
M. Bensitel, O. Saur, J.-C. Lavalley and G. Mabilon, Mater. Chem. Phys. 17 (1987) 249.
M. Bensitel, O. Saur, J.-C. Lavalley and B.A. Morrow, Mater. Chem. Phys. 19 (1988) 147.
V.S. Komarov and M.F. Sinilo, Kinet. Katal. 29 (1988) 701.
M. Waqif, J. Bachelier, O. Saur and J.-C. Lavalley, J. Mol. Catal. 72 (1992) 127.
C. Morterra, G. Cerrato and V. Bolis, Catal. Today 17 (1993) 505.
A. Clearfield, G.D.P. Serrette and A.H. Khazi-Syed, Catal. Today 20 (1994) 295.
L.M. Kustov, V.B. Kazansky, F. Figueras and D. Tichit, J. Catal. 150 (1994) 143.
T. Riemer, D. Spielbauer, M. Hunger, G.A.H. Mekhemer and H. Knözinger, J. Chem. Soc. Chem. Commun. (1994) 1181.
V. Adeeva, J.W. de Haan, J. Jänchen, G.D. Lei, V. Schünemann, L.J.M. van de Veen, W.H.M. Sachtler and R.A. van Santen, J. Catal. 151 (1995) 364.
F. Babou, G. Coudurier and J.C. Vedrine, J. Catal. 152 (1995) 341.
R.L. White, E.C. Sikabwe, M.A. Coelho and D.E. Resasco, J. Catal. 157 (1995) 755.
E.E. Platero, M.P. Mentruit, C.O. Arean and A. Zecchina, J. Catal. 162 (1996) 268.
A.A. Tsyganenko and V.N. Filiminov, J. Mol. Struct. 19 (1973) 579.
F. Haase and J. Sauer, J. Amer. Chem. Soc. 120 (1998) 13503.
C. Morterra, G. Cerrato, F. Pinna, M. Signoretto and G. Strukul, J. Catal. 149 (1994) 181.
C. Morterra and G. Cerrato, Phys. Chem. Chem. Phys. 1 (1999) 2825.
P.J. Wagner, M.A. Meador and B.-S. Park, J. Amer. Chem. Soc. 112 (1990) 5199.
H.O. Kalinowski, S. Berger and S. Braun, 13 C-NMR-Spektroskopie (Georg Thieme Verlag, Stuttgart-New York, 1984) p. 285.
D.D. Wirth, Tetrahedron 49 (1993) 1535.
I. Ahmad, J.A. Anderson, C.H. Rochester and T.J. Dines, J. Mol. Catal. A 135 (1998) 63.
R. Steward and K. Yates, J. Am. Chem. Soc. 80 (1958) 6355.
B.S. Umansky and W.K. Hall, J. Catal. 124 (1990) 97.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trunschke, A., Deutsch, J., Müller, D. et al. Nature of Surface Deposits on Sulfated Zirconia Used as Catalyst in the Benzoylation of Anisole. Catalysis Letters 83, 271–279 (2002). https://doi.org/10.1023/A:1021050401227
Issue Date:
DOI: https://doi.org/10.1023/A:1021050401227