Abstract
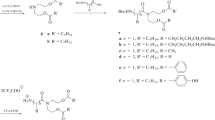
Nine fatty acid–peptide hybrid molecules were constructed using the general formula CH3(CH2)nCO-Phe Asp Cys-amide and tested for their ability to inhibit cell lysis induced by the membrane-active peptide melittin. All of these molecules, where n = 4–14, inhibited the action of melittin to some extent, but the longer carbon chains were most effective. Several potential inhibitors were also constructed with conservative substitutions in the peptide portion of the molecule. All were effective to varying degrees. We concluded that in the hexapeptide inhibitor published by Blondelle et al. (1993), the role of the first three residues is only to provide hydrophobic interaction with the melittin and has no particular amino acid sequence specificity. Some of these inhibitors were found to inhibit the lytic activity of a melittin analogue which had only superficial sequence similarity to melittin and also a truncated form of melittin, indicating the generality of the action of the inhibitors.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Deceased 5/4/98
Rights and permissions
About this article
Cite this article
Rivett, D.E., Hewish, D., Kirkpatrick, A. et al. Inhibition of Membrane-Active Peptides by Fatty Acid–Peptide Hybrids. J Protein Chem 18, 291–295 (1999). https://doi.org/10.1023/A:1021035328105
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1021035328105