Abstract
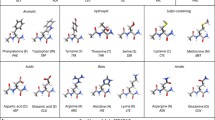
The chemical shift difference (δ[13Cβ] − δ[13Cγ]) is a reference-independent indicator of the Xaa-Pro peptide bond conformation. Based on a statistical analysis of the 13C chemical shifts of 1033 prolines from 304 proteins deposited in the BioMagRes database, a software tool was created to predict the probabilities for cis or trans conformations of Xaa-Pro peptide bonds. Using this approach, the conformation at a given Xaa-Pro bond can be identified in a simple NOE-independent way immediately after obtaining its NMR resonance assignments. This will allow subsequent structure calculations to be initiated using the correct polypeptide chain conformation.
Similar content being viewed by others
References
Badger, J., Kumar, R.A., Yip, P. and Szalma, S. (1999) Proteins, 35, 25–33.
Berman, H.M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T.N., Weissig, H., Shindyalov, I.N. and Bourne, P.E. (2000) Nucl. Acids Res., 28, 235–242.
Bernstein, F.C., Koetzle, T.F., Williams, G.J.B., Meyer, E.F., Brice, M.D., Rodgers, J.R., Kennard, O. Shimanouchi, T. and Tasumi, M. (1977) J. Mol. Biol., 112, 535–542.
Bronstein, I.N. and Semendjajew, K.A. (1985) Taschenbuch der Mathematik, Teubner Verlag, Leipzig.
Brünger, A.T.(1992) X-Plor, Version 3.1: A system for X-Ray Crystallography and NMR, Yale University Press, New Haven.
Dorman, D.E. and Bovey, F.A. (1973) J. Org. Chem., 38, 2379–2383 and references cited therein.
Giessner-Prettre, C., Cung, M.T. and Marraud, M. (1987)Eur. J. Biochem., 163, 79–87.
Haasnoot, C.A.G., de Leeuw, F.A.A.M., deLeeuw, H.P.M. and Altona, C. (1981) Biopolymers, 20, 1211–1245.
Herrmann, T., Güntert, P. and Wüthrich, K. (2002) J. Mol. Biol., 319, 209–227.
Jabs, A., Weiss, M.S.and Hilgenfeld, R. (1999) J. Mol. Biol., 286, 291–304.
Kang, Y.K. (1996) J. Phys.Chem., 100, 11589–11595.
Linge, J.P., O'Donoghue, S.I. and Nilges, M. (2001) Meth.Enzymol., 339, 71–90.
London, R.E. (1978) J. Am. Chem. Soc., 100, 2678–2685.
Milner-White, E.J., Bell, L.H. and Maccallum, P.H. (1992) J. Mol. Biol., 228, 725–734.
Güntert, P., Mumenthaler, C. and Wüthrich, K. (1997) J. Mol. Biol., 273, 283–298.
Nilges, M., Macias, M.J., O'Donoghue, S.I. and Oschkinat, H. (1997) J. Mol. Biol., 269, 408–422.
Ramachandran, G. N. and Sasisekharan, V. (1968) Adv. Prot. Chem., 23,283–437.
Schmidt, J.M., Brüschweiler, R., Ernst, R.R., Dunbrack, R.L., Joseph, D. and Karplus, M. (1993) J. Am. Chem. Soc., 115, 8747–8756.
Seavey, B.R., Farr, E.A., Westler, W.M. and Markley, J.L. (1991) J. Biomol. NMR, 1, 217–236.
Siemion, I.Z., Wieland, T. and Pook, K.-H. (1975) Angew. Chem., 87, 712–714.
Stanczyk, S.M., Bolton, P.H., Dell'Acqua, M. and Gerlt, J.A. (1989) J. Am. Chem. Soc., 111, 8317–8318.
Torchia, D.A., Sparks, S.W., Young, P.E. and Bax, A. (1989) J. Am. Chem. Soc., 111,8315–8317.
Weiss, M.S., Jabs, A. and Hilgenfeld, R. (1998) Nat. Struct. Biol., 5,676.
Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, Wiley, New York, NY.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schubert, M., Labudde, D., Oschkinat, H. et al. A software tool for the prediction of Xaa-Pro peptide bond conformations in proteins based on 13C chemical shift statistics. J Biomol NMR 24, 149–154 (2002). https://doi.org/10.1023/A:1020997118364
Issue Date:
DOI: https://doi.org/10.1023/A:1020997118364