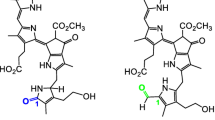
Abstract
In extracts of senescent leaves of spinach (Spinacia oleracea), five colourless compounds with UV/Vis-characteristics of nonfluorescent chlorophyll catabolites (NCCs) were detected and tentatively named So-NCCs. The most abundant polar NCC in the leaves of this vegetable, So-NCC-2, had been isolated earlier and its constitution was determined by spectroscopic means. The catabolite So-NCC-2 was found to be an epimer of a polar NCC from barley (Hordeum vulgare), the first non-green chlorophyll catabolite from a higher plant to be structurally analyzed. Here, we report on the isolation of four additional So-NCCs from the extracts of senescent leaves of Sp. oleracea by two- (or multi-)stage chromatographic purification and on their structural characterization. The constitution of So-NCC-3 could be determined by spectroscopic analysis in combination with chemical correlation with a known NCC from Cercidiphyllum japonicum (Cj-NCC): So-NCC-3 was identified as the hydrolysis product of the methyl ester function of Cj-NCC. The less polar catabolite So-NCC-4 could be directly identified with Cj-NCC. Two further So-NCCs, So-NCC-1 and So-NCC-5, were detected only in trace amounts. Five structurally related nonfluorescent chlorophyll catabolites (So-NCCs) are thus present in senescent leaves of spinach. The structures of this set of So-NCCs indicate several peripheral refunctionalization reactions and inform on the late catabolic transformations during leaf senescence. The transformation of the tetrapyrrolic skeleton in chlorophyll catabolism in spinach and in C. japonicum is revealed to exhibit a common stereochemical pattern.
Similar content being viewed by others
References
Bortlik K-H, Peisker C and Matile P (1990) A novel type of chlorophyll catabolite in senescent barley leaves. J Plant Physiol 136: 161–165
Brown SB, Houghton JD and Hendry GAF (1991) Chlorophyll breakdown. In:Scheer H (ed) Chlorophylls, pp 465–489. CRC Press, Boca Raton, Florida
Curty C and Engel N (1996) Detection, isolation and structure elucidation of a chlorophyll a catabolite from autumnal senescent leaves of Cercidiphyllum japonicum. Phytochemistry 42: 1531–1536
Doi M, Inage T and Shioi Y (2001) Chlorophyll degradation in a Chlamydomonas reinhardtii mutant: an accumulation of pyropheophorbide a by anaerobiosis. Plant Cell Physiol 42: 469–474
Ginsburg S and Matile P (1993) Identification of catabolites of chlorophyll porphyrin in senescent rape cotyledons. Plant Physiol 102: 521–527
Hendry GAF, Houghton JD and Brown SB (1987) The degradation of chlorophyll-a biological enigma. New Phytol 107: 255–302
Hinder B, Schellenberg M, Rodoni S, Ginsburg S, Vogt E, Martinoia E, Matile P and Hörtensteiner S (1996) How plants dispose of chlorophyll catabolites. Directly energized uptake of tetrapyrrolic breakdown products into isolated vacuoles. J Biol Chem 271: 27233–27236
Hörtensteiner S (1998a) NCC malonyltransferase catalyses the fi-nal step of chlorophyll breakdown in rape (Brassica napus). Phytochemistry 49: 953–956
Hörtensteiner S and Kräutler B (2000) Chlorophyll breakdown in oilseed rape. Photosynth Res 64: 137–146
Hörtensteiner S, Vicentini F and Matile P (1995) Chlorophyll breakdown in senescent cotyledons of rape, Brassica napus L.: enzymatic cleavage of phaeophorbide a in vitro. New Phytol 129: 237–246
Hörtensteiner S, Wüthrich KL, Matile P, Ongania K-H and Kräutler B (1998b) The key step in chlorophyll breakdown in higher plants. Cleavage of pheophorbide a macrocycle by a monooxygenase. J Biol Chem 273: 15335–15339
Hörtensteiner S, Rodoni S, Schellenberg M, Vicentini F, Nandi OI, Qiu Y-L and Matile P (2000) Evolution of chlorophyll degradation: the significance of RCC reductase. Plant Biol 2: 63–67
Ito H, Tanaka Y, Tsuji H and Tanaka A (1993) Conversion of chlorophyll b to chlorophyll a by isolated cucumber etioplasts. Arch Biochem Biophys 306: 148–151
Ito H, Ohysuka T and Tanaka A (1996) Conversion of chlorophyll b to chlorophyll a via 7-hydroxymethyl chlorophyll. J Biol Chem 271: 1475–1479
Iturraspe J, Moyano N and Frydman B (1995) A new 5-formylbilinone as the major chlorophyll a catabolite in tree senescent leaves. J Org Chem 60: 6664–6665
Kessler H, Gehrke M and Griesinger C (1988) Zweidimensionale NMR-Spektroskopie, Grundlagen und Ñbersicht über die Experimente. Angew Chem 100: 507–544; Angew Chem Int Ed Engl 27: 490-537
Kräutler B and Matile P (1999) Solving the riddle of chlorophyll breakdown. Acc Chem Res 32: 35–43
Kräutler B, Jaun B, Bortlik K-H, Schellenberg M and Matile P (1991) On the enigma of chlorophyll degradation: the constitution of a secoporphinoid catabolite. Angew Chem Int Ed Engl 30: 1315–1318
Kräutler B, Jaun B, Amrein W, Bortlik K, Schellenberg M and Matile P (1992) Breakdown of chlorophyll: constitution of a secoporphinoid chlorophyll catabolite isolated from senescent barley leaves. Plant Physiol Biochem 30: 333–346
Losey FG and Engel N (2001) Isolation and characterization of a urobilinogenoidic chlorophyll catabolite from Hordeum vulgare. J Biol Chem 276: 8643–8647
Lu Y-P, Li Z-S, Drozdowicz Y-M, Hörtensteiner S, Martinoia E and Rea PA (1998) AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with AtMRP1. Plant Cell 10: 267–282
Matile P (1987) Seneszenz bei Pflanzen und ihre Bedeutung für den Stickstoffhaushalt. Chimia 41: 376–381
Matile P and Kräutler B (1995) Wie und warum bauen Pflanzen das Chlorophyll ab. Chem unserer Zeit 29: 298–306
Matile P, Ginsburg S, Schellenberg M and Thomas H (1988) Catabolites of chlorophyll in senescing barley leaves are localized in the vacuoles of mesophyll cells. Proc Natl Acad Sci USA 85: 9529–9532
Matile P, Hörtensteiner S, Thomas H and Kräutler B (1996) Chlorophyll breakdown in senescent leaves. Plant Physiol 112: 1403–1409
Mendel G (1865) Versuche über Pflanzenhybriden. Verhandlungen des Naturwissenschaftlichen Vereins, Brünn 4: 3–47
Mühlecker W and Kräutler B (1996) Breakdown of chlorophyll: constitution of nonfluorescing chlorophyll-catabolites from senescent cotyledons of the dicot rape. Plant Physiol Biochem 34: 61–75
Mühlecker W, Kräutler B, Ginsburg S and Matile P (1993) Breakdown of chlorophyll: the constitution of a secoporphinoid chlorophyll catabolite from senescent rape leaves. Helv Chim Acta 76: 2976–2980
Mühlecker W, Ongania K-H, Kräutler B, Matile P and Hörtensteiner S (1997) Tracking down chlorophyll breakdown in plants: elucidation of the constitution of a fluorescent chlorophyll catabolite. Angew Chem Int Ed Engl 36: 401–404
Mühlecker W, Kräutler B, Moser D, Matile P and Hörtensteiner S (2000) Breakdown of chlorophyll: a fluorescent chlorophyll catabolite from sweet pepper (Capsicum annuum). Helv Chim Acta 83: 278–286
Oberhuber M, Berghold J, Mühlecker W, Hörtensteiner S and Kräutler B (2001) Chlorophyll breakdown-on a nonfluorescent chlorophyll catabolite from spinach. Helv Chim Acta 84: 2615–2627
Pretsch E, Bühlmann P and Affolter C (2000) Structure Determination of Organic Compounds, pp 158–160. Springer-Verlag, Berlin
Rodoni S, Vicentini F, Schellenberg M, Matile P and Hörtensteiner S (1997) Partial purification and characterization of red chlorophyll catabolite reductase, a stroma protein involved in chlorophyll breakdown. Plant Physiol 115: 677–682
Sanders JKM and Hunter BK (1987) Modern NMR-spectroscopy. Oxford University Press, Oxford Scheer H (ed) (1991) Chlorophylls. CRC-Press, Boca Raton, Florida
Scheumann V, Ito H, Tanaka A, Schoch S and Rüdiger W (1996) Substrate specificity of chlorophyll(ide) b reductase in etioplasts of barley (Hordeum vulgare). Eur J Biochem 242: 163–170
Scheumann V, Schoch S and Rüdiger W (1999) Chlorophyll b reduction during senescence of barley seedlings. Planta 209: 364–370
Shioi Y, Watanabe K and Takamiya K (1996) Enzymatic conversion of pheophorbide a to a precursor of pyropheophorbide a in leaves of Chenopodium album. Plant Cell Physiol 37: 1143–1149
Smith RD, Light-Wahl KJ, Winger BE and Goodlett DR (1995) Electrospray Ionization. In: Matsuo T, Caprioli RM, Gross ML and Seyama Y (eds) Biological Mass Spectrometry: Present and Future, pp 41–74. John Wiley & Sons, Chichester, UK
Tommasini R, Vogt E, Fromenteau M, Hörtensteiner S, Matile P, Amrhein N and Martinoia E (1998) An ABC transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J 13: 773–780
Watson TR (1995) Fast atom bombardment. In: Matsuo T, Caprioli RM, Gross ML and Seyama Y (eds) Biological Mass Spectrometry: Present and Future, pp 24–40. John Wiley & Sons, Chichester, UK
Wüthrich KL, Bovet L, Hunziker PE, Donnison IS and Hörtensteiner S (2000) Molecular cloning, functional expression and characterisation of RCC reductase involved in chlorophyll catabolism. Plant J 21: 189–198
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berghold, J., Breuker, K., Oberhuber, M. et al. Chlorophyll breakdown in spinach: on the structure of five nonfluorescent chlorophyll catabolites* . Photosynthesis Research 74, 109–119 (2002). https://doi.org/10.1023/A:1020991023248
Issue Date:
DOI: https://doi.org/10.1023/A:1020991023248