Abstract
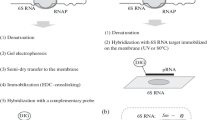
With the object of elucidating the factors that affect the rate and efficiency of oligonucleotide hybridization with complementary regions in natural RNA, the interaction of tRNAPhe and in vitro transcript of this tRNA with synthetic oligonucleotides matching the 32—61 region comprising the variable loop and anticodon and TΨC stems of the molecule was studied in detail. Gel retardation was used to assay thermodynamic and kinetic parameters of tRNA interaction with oligonucleotides. Oligonucleotide hybridization rates do not depend on the oligonucleotide length and are determined by the stability of RNA structure within the binding site. The rate constant for hybridization correlates with the number of base pairs in the RNA opened during binding with the oligonucleotide. Factors stabilizing the tRNA secondary structure (the presence of Mg2+ ions, low temperature, hypermodified bases) decrease significantly both the rate and efficiency of hybridization. Analysis of the tRNAPhe structure in the presence of an oligonucleotide shows that binding of oligonucleotides with the 45—61 region of tRNAPhe results only in unfolding of the TΨC hairpin and does not disturb the secondary structure of the rest of the molecule.
Similar content being viewed by others
References
S. T. Crooke, Methods Enzymol., 2000, 313, 3.
D. J. Ecker, in Antisense research and applications, Eds. S. T. Crooke and B. Lebleau, CRC Press, Boca Raton, 1993, 387.
S. Baskerville and A. D. Ellington, Curr. Biol., 1995, 5, 120.
R. T. Batey, R. P. Rambo, and J. A. Doudna, Angew. Chem. Int., Ed. Engl., 1999, 38, 2326.
A. D. Branch, Trends Biochem. Sci., 1998, 23, 45.
R. A. Stull, G. Zon, and F. C. Szoka, Jr., Antisense Nucleic Acid Drug. Dev., 1996, 6(3), 221.
K. U. Mir and E. M. Southern, Nature Biotechnol., 1999, 17, 788.
Y. Kumazawa, T. Yokogawa, H. Tsurui, K. Miura, and K. Watanabe, Nucleic Acids Res., 1992, 20, 2223.
T. Kanda, K. Takai, S. Yokoyama, and H. Takaku, Bioorg. Med. Chem., 2000, 8, 675.
V. Petyuk, R. Serikov, V. Tolstikov, V. Potapov, R. Giege, M. Zenkova, and V. Vlassov, Nucleosides, Nucleotides Nucleic Acids, 2000, 19, 1145.
V. Petuyk, M. Zenkova, R. Giege, and V. V. Vlassov, FEBS Letters, 1999, 444, 217.
V. A. Petyuk, M. A. Zenkova, R. Giege, and V. V. Vlassov, Molekulyar. Biol., 2000, 34, 879 [Mol. Biol., 2000, 34 (Engl. Transl.)].
J. F. Milligan and O. C. Uhlenbeck, Methods Enzymol., 1989, 180, 51.
J. R. Sampson and O. C. Uhlenbeck, Proc. Natl. Acad. Sci. USA, 1988, 85, 1033.
T. E. England, A. G. Bruce, and O. C. Uhlenbeck, Methods Enzymol., 1980, 65, 65.
D. Lane, P. Prentki, and M. Chandler, Microbiol. Rev., 1992, 56(4), 509.
J. Meador, B. Cannon, V. J. Cannistraro, and D. Kennell, Eur. J. Biochem., 1990, 187, 549.
M. I. Dobrikov, S. A. Gaidamakov, A. A. Koshkin, T. I. Guinutdinov, N. P. Luk´yanchuk, G. V. Shishkin, and V. V. Vlassov, Bioorgan. Khim., 1997, 23, 191 [Russ. J. Bioorg. Chem., 1997, 23 (Engl. Transl.)].
M. Sprinzl, Eur. J. Biochem., 1974, 49, 595.
J. D. Robertus, J. E. Ladner, J. T. Finch, D. Rhodes, R. S. Brown, B. F. Clark, and A. Klug, Nature, 1974, 250, 546.
O. C. Uhlenbeck, J. Mol. Biol., 1972, 65, 25.
N. Sugimoto, S. Nakano, M. Katoh, A. Matsumura, H. Nakamuta, T. Ohmichi, M. Yoneyama, and M. Sasaki, Biochemistry, 1995, 34, 11211.
M. E. Craig, D. M. Crothers, P. Doty, J. Mol. Biol., 1971, 62, 383.
V. Patzel and G. Szakiel, Nucleic Acids Res., 2000, 13, 2462.
C. R. Cantor and P. R. Schimmel, in Biophysical Chemistry, Part 3: The Behavior of Biological Macromolecules, W. H. Freeman, San Francisco, 1980, 3, 23.
O. Pongs and E. Reinwald, Biochem. Biophys. Res. Commun., 1973, 50, 357.
P. Narayan and F. Ramirez, Biochim. Biophys. Acta, 1978, 518, 539.
P. F. Agris, A. Malkiewicz, R. Guenther, M. Basti, R. Sengupta, and J. Stuart, Nucleic Acids Symp. Ser., 1995, 33, 254.
M. Homann, K. Rittner, and G. Sczakiel, J. Mol. Biol., 1993, 233, 7.
K. Rittner, C. Burmester, and G. Sczakiel, Nucleic Acids Res., 1993, 21, 1381.
R. Kronenwett, R. Haas, and G. Sczakiel, J. Mol. Biol., 1996, 259, 632.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Serikov, R.N., Petyuk, V.A., Vlassov, V.V. et al. Hybridization of antisense oligonucleotides with yeast tRNAPhe: factors determining the efficiency of interaction. Russian Chemical Bulletin 51, 1156–1165 (2002). https://doi.org/10.1023/A:1020975724157
Issue Date:
DOI: https://doi.org/10.1023/A:1020975724157