Abstract
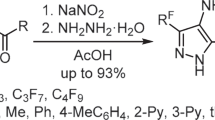
The reaction of 2-hydroxy-2-polyfluoroalkylchroman-4-ones with hydrazine affords 5-hydroxy-3-(2-hydroxyaryl)-5-polyfluoroalkyl-Δ2-pyrazolines, whereas 2-polyfluoroalkylchromones under similar conditions produce 3(5)-(2-hydroxyaryl)-5(3)-polyfluoroalkylpyrazoles. 5-(2-Hydroxyaryl)-1-methyl-3-polyfluoroalkylpyrazoles were synthesized in the reaction with methylhydrazine, and the reaction with phenylhydrazine afforded regioisomeric 3(5)-(2-hydroxyphenyl)-1-phenyl-5(3)-polyfluoroalkylpyrazoles.
Similar content being viewed by others
References
V. Ya. Sosnovskikh, A. Yu. Sizov, and B. I. Usachev, Izv. Akad. Nauk, Ser. Khim., 2002, 1175 [Russ. Chem. Bull., Int. Ed., 2002, 51, 1270].
J. Catalán, F. Fabero, R. M. Claramunt, M. D. Santa Maria, M. C. Foces-Foces, F. H. Cano, M. Martinez-Ripoll, J. Elguero, and R. Sastre, J. Am. Chem. Soc., 1992, 114, 5039.
J. Catalán, J. C. del Valle, R. M. Claramunt, M. D. Santa Maria, V. Bobosik, R. Mocelo, and J. Elguero, J. Org. Chem., 1995, 60, 3427.
S. R. Bertenshaw, J. J. Talley, D. J. Rogier, M. J. Graneto, C. M. Koboldt, and Y. Zhang, Bioorg. Med. Chem. Lett., 1996, 6, 2827.
T. D. Penning, J. J. Talley, S. R. Bertenshaw, J. S. Carter, P. W. Collins, S. Docter, M. J. Graneto, L. F. Lee, J. W. Malecha, J. M. Miyashiro, R. S. Rogers, D. J. Rogier, S. S. Yu, G. D. Anderson, E. G. Burton, J. N. Cogburn, S. A. Gregory, C. M. Koboldt, W. E. Perkins, K. Seibert, A. W. Veenhuizen, Y. Y. Zhang, and P. C. Isakson, J. Med. Chem., 1997, 40, 1347.
X.-Q. Tang and C.-M. Hu, J. Chem. Soc., Perkin Trans. 1, 1994, 2161.
D. V. Sevenard, O. G. Khomutov, M. I. Kodess, K. I. Pashkevich, I. Loop, E. Lork, and G.-V. Röschenthaler, Can. J. Chem., 2001, 79, 183.
V. Ya. Sosnovskikh and B. I. Usachev, Izv. Akad. Nauk, Ser. Khim., 2001, 1357 [Russ. Chem. Bull., Int. Ed., 2001, 50, 1426].
V. Ya. Sosnovskikh, I. I. Vorontsov, and V. A. Kutsenko, Izv. Akad. Nauk, Ser. Khim., 2001, 1360 [Russ. Chem. Bull., Int. Ed., 2001, 50, 1430].
V. Ya. Sosnovskikh, B. I. Usachev, and M. I. Kodess, Izv. Akad. Nauk, Ser. Khim., 2002, No. 10 [Russ. Chem. Bull., Int. Ed., 2002, 51, No. 10].
V. Ya. Sosnovskikh, B. I. Usachev, and A. P. Safronov, Izv. Akad. Nauk, Ser. Khim., 2001, 1253 [Russ. Chem. Bull., Int. Ed., 2001, 50, 1314].
K. N. Zelenin and S. I. Yakimovitch, Targets in Heterocyclic Systems, 1998, 2, 207.
C. Massyn and A. Cambon, J. Fluorine Chem., 1975, 5, 67.
V. Ya. Sosnovskikh, M. Yu. Mel´nikov, A. V. Zaitsev, and E. A. Bogdanov, Izv. Akad. Nauk, Ser. Khim., 1998, 1201 [Russ. Chem. Bull., 1998, 47, 1170 (Engl. Transl.)].
J. L. Peglion, R. E. Pastor, and A. R. Cambon, Bull. Soc. Chim. Fr., 1980, II-309.
S. P. Singh, D. Kumar, and M. D. Threadgill, Ind. J. Chem., Sect. B, 1992, 31, 233.
S. P. Singh, J. K. Kapoor, D. Kumar, and M. D. Threadgill, J. Fluor. Chem., 1997, 83, 73.
A. B. Denisova, T. V. Glukhareva, G. P. Andronnikova, V. S. Mokrushin, W. Dehaen, I. Luyten, V. Y. Sosnovskikh, L. Meervelt, and V. A. Bakulev, J. Chem. Res. (S), 2001, 12; J. Chem. Res. (M), 2001, 0133.
S. P. Singh, D. Kumar, B. G. Jones, and M. D. Threadgill, J. Fluor. Chem., 1999, 94, 199.
K. N. Zelenin, V. V. Alekseyev, A. R. Tygysheva, and S. I. Yakimovitch, Tetrahedron, 1995, 51, 11251.
E. Morera and G. Ortar, Tetrahedron Lett., 1981, 22, 1273.
W. Baker, J. B. Harborne, and W. D. Ollis, J. Chem. Soc., 1952, 1303.
V. K. Ahluwalia, D. Kumar, N. Rani, and Sunita, Ind. J. Chem., Sect. B, 1977, 15, 328.
V. K. Ahluwalia, D. Kumar, and M. C. Gupta, Ind. J. Chem., Sect. B, 1978, 16, 216.
C. Morin and R. Beugelmans, Tetrahedron, 1977, 33, 3183.
L. G. Grishko, V. P. Khilya, M. F. Sedyuko, and D. Litkei, Ukr. Khim. Zh. [Ukranian Chem. J.], 1985, 51, 211 (in Russian).
V. Ya. Sosnovskikh and B. I. Usachev, Mendeleev Commun., 2000, 240.
J. K. Williams, J. Org. Chem., 1964, 29, 1377.
F. Aguilar-Parrilla, C. Cativiela, M. D. Diaz de Villegas, J. Elguero, C. Foces-Foces, J. I. G. Laureiro, F. H. Cano, H.-H. Limbach, J. A. S. Smith, and C. Toiron, J. Chem. Soc., Perkin Trans. 2, 1992, 1737.
J. Elguero, G. I. Yranzo, J. Laynez, P. Jiménez, M. Menéndez, J. Catalán, J. L. G. de Paz, F. Anvia, and R. W. Taft, J. Org. Chem., 1991, 56, 3943.
V. Ya. Sosnovskikh, Izv. Akad. Nauk, Ser. Khim., 2001, 1166 [Russ. Chem. Bull., Int. Ed., 2001, 50, 1223].
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sosnovskikh, V.Y., Barabanov, M.A. & Sizov, A.Y. 2-Polyfluoroalkylchromones. 11. Synthesis and structures of 5-hydroxy-3-(2-hydroxyaryl)-5-polyfluoroalkyl-Δ2-pyrazolines and 3(5)-(2-hydroxyaryl)-5(3)-polyfluoroalkylpyrazoles. Russian Chemical Bulletin 51, 1280–1291 (2002). https://doi.org/10.1023/A:1020960815497
Issue Date:
DOI: https://doi.org/10.1023/A:1020960815497