Abstract
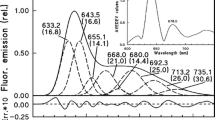
A hypothesis describing the mechanism of photoactive protochlorophyllide (P) photoreduction in vivo, relating mainly to the molecular nature of the intermediates, is proposed. The hypothesis is compatible with currently published experimental data. After illumination of etiolated barley leaves at 143 to 153 K, the absorption of P remains essentially unchanged, but a new absorption band at 690 nm is observed. Appearance of this new intermediate enables to distinguish between light and dark stages of the photoconversion reaction. When returned to the higher temperature in the dark, the treated leaves begin accumulating chlorophyllide (Chlide), concomitant with the disappearance of the 690-nm band. The decay time of the excited P (P*) is estimated at 300 ps, which approximates the time constant of photoinduced electron transfer (ET). It is suggested that the charge-transfer complex (CTC) in its ground state (GS) (ground state of CTC formed by the partial (δ) electron transfer), i.e. (Pδ−•••H–Dδ+), between P and NADPH – the electron and proton donor (H–D) – accumulates in the following sequence: P* + H–D → (P*•••H–D)→[(P*•••H–D)←(P−•••H–D+)] → 1(P−•••H–D+)] → 3(P−•••H–D+) → (Pδ−•••H–D δ+), where an equilibrium state (ES) – [(P*•••H–D)←(P−•••H–D+)] – with a lifetime of about 1 to 2 ns, exists between the local excited (LE) and ET states. The existence of a triplet ET state – 3(P−•••H–D+) – is proposed because the time interval between recording of the ES and appearance of the CTC GS (35–250 ns) does not fit the lifetime of the singlet excited complex (exciplex). It is feasible that apart from NADPH, other intermediate proton carriers are contemporaneously involved in the dark reaction (Pδ−•••H–Dδ+) → Chlide, because proton binding to the C7–C8 bond in vivo takes place in the trans-configuration. The hydride ion may approach the C7–C8 bond from one side by heterolytic fission and an additional proton, donated by the protein group, may be simultaneously added to this bond from the opposite side of the porphyrin nucleus surface.
Similar content being viewed by others
References
Allison JB and Becker RS (1963) Metalloporphyrins. III. Electronic spectra and nature of perturbation: absorption spectra and solutesolvent interactions. J Phys Chem 67: 2675–2679
Beer NS and Griffith WT (1981) Purification of the enzyme NADPH: protochlorophyllide oxidoreductase. Biochem J 195: 83–92
Begley TP and Young H (1989) Protochlorophyllide reductase. 1. Determination of the regiochemistry and the stereochemistry of the reduction of protochlorophyllide to chlorophyllide. J Am Chem Soc 111: 3095–3096
Belyaeva OB, Timofeev KN and Litvin FF (1988) The primary reactions in the protochlorophyll(ide) photoreduction as investigated by optical and ESR spectroscopy. Photosynth Res 15: 247–256
Birks JB (1970) In: Birks JB (ed) Photophysics of Aromatic Molecules, pp 301–491. Wiley-Interscience, London/New York/Sydney/Toronto
Bistrova MI, Umrihina AV and Krasnovsky AA (1966) Protochlorophyll and protopheophytin reduction. Biochemistry (Moscow) 31: 83–91 [in Russian]
Blankenhorn G (1976) Nicotinamide-dependent one-electron and two-electron (flavin) oxidoreduction: thermodynamics, kinetics and mechanism. Eur J Biochem 67: 67–80
Bonnett R, Lambert C, Land EJ, Scourides PA, Sinclair RS and Truscott TG (1983) The triplet and radical species of haematoporphyrin and some of its derivatives. Photochem Photobiol 38: 1–8
Chang CJ, Brown JDK, Chang MCY, Baker EA and Nocera DG (2001) Electron transfer in hydrogen-bonded donor-acceptor supramolecules. In: Balzani V (ed) Electron Transfer in Chemistry, Vol 3, pp 409–461. Wiley-YCH Verlag, Weinheim
Dodd JW and Hush NS (1964) The negative ions of some porphin and phthalocyanine derivatives, and their electronic spectra. J Chem Soc 86: 4607–4614
Dujardin E and Sironval C (1977) Transitory pigment-protein complexes similar to photosynthesis active centers during protochlorophyll(ide) photoreduction. Plant Sci Lett 10: 347–355
Franck F and Mathis P (1980) A short-lived intermediate in the photoenzymatic reduction of protochlorophyll(ide) into chlorophyll( ide) at a physiological temperature. Photochem Photobiol 32: 799–803
Frank SR (1946) The effectiveness of the spectrum in chlorophyll formation. J Gen Physiol 29: 157–179
Goedheer JC and Verhulsdonk CAH (1970) Fluorescence and phototransformation of protochlorophyll with etiolated bean leaves from-196 to +20 °C. Biochem Biophys Res Comm 39: 260–266
Griffiths WT (1974) Protochlorophyll and protochlorophyllide as precursors for chlorophyll synthesis in vivo. FEBS Lett 49: 196–200
Griffiths WT (1991) Protochlorophyllide photoreduction. In: Scheer H (ed) Chlorophylls, pp 433–449. CRC Press, Boca Raton, Florida
Iwai J, Ikeuchi M, Inoue Y and Kobajashi T (1984) Early processes of protochlorophyllide photoreduction as measured by nanosecond and picosecond spectrophotometry. In: Sironval C and Brouers M (eds) Protochlorophyllide Reduction and Greening, pp 165–178. Nijhoff and Junk Publishers, The Hague
Krasnovsky AA, Jr, Belyaeva OB, Kovalev YV, Ignatov NV and Litvin FF (1999) Phosphorescence of intermediates of the terminal stage of chlorophyll biosynthesis in plants. Biochemistry (Moscow) 64: 587–591
Lebedev N and Timko MP (1999) Protochlorophyllide oxidoreductase B-catalyzed protochlorophyllide photoreduction in vitro: insight into the mechanism of chlorophyll formation in light-adapted plants. Proc Natl Acad Sci USA 96: 9954–9959
Marcus RA and Sutin N (1985) Electron transfer in chemistry and biology. Biochim Biophys Acta 811: 265–322
Mataga N (1997) Development of exciplex chemistry: some fundamental aspects. Pure Appl Chem 69: 729–734
Mataga N, Karen A, Okada T, Nishitani S, Kurata N, Sakata Y and Misumi S (1984) Picosecond dynamics of photochemical electron transfer in porphyrin-quinone intramolecular exciplex systems. J Phys Chem 88: 5138–5141
Mauzerall D and Feher G (1964) Optical absorption of the porphyrin free radical formed in a reversible photochemical reaction. Biochim Biophys Acta 88: 658–660
Miyasaka H, Tabata A, Kamada K and Mataga N (1993a) Femtosecond-picosecond laser photolysis studies on the mechanism of electron transfer induced by hydrogen-bonding interactions in nonpolar solutions: 1-aminopyrene-pyridine systems. J Am Chem Soc 115: 7335–7342
Miyasaka H, Tabata A, Ojiama S, Ikeda N and Mataga N (1993b) Femtosecond-picosecond laser photolysis studies on the mechanism of fluorescence quenching induced by hydrogen-bonding interactions-1-pyrenol-pyridine systems. J Phys Chem 97: 8222–8228
Nikolaeva LF, Raskin VI and Zakirov A (1972) Efficiency of photoreduction and transformation of protochlorophyllide forms in the presence of nicotinamide adenine dinucleotide phosphate. Biologicheskiye Nauki (Biological Sciences), Moscow 4: 70–74 [in Russian]
Raskin VI (1976) Mechanism of protochlorophyllide photoreduction in intact etiolated leaves. Vestsi Acad Nauk BSSR, Ser Biol Sciences 5: 43–46 [in Russian]
Raskin VI (1979) Change in quantum yield of chlorophyllide fluorescence during reduction of protochlorophyllide in etiolated leaves. Doklady Biophysics. Proceedings of the Academy of Sciences of the USSR. Biophysics Section 245: 100–102 [translated from Russian]
Raskin VI (1981) Protochlorophyllide Photoreduction. Kaler VL (ed). Science and Technique, Minsk [in Russian]
Raskin VI, Nikolaeva LF, Pashchenko VZ, Tusov VB and Rubin AB (1985) The rate of intermediate formation in the reaction of protochlorophyllide photoreduction in intact leaves. Biologicheskiye Nauki (Biological Sciences), Moscow 7: 26–28 [in Russian]
Rubin AB, Minchenkova LE, Krasnovsky AA and Tummerman LA (1962)] Investigation of average protochlorophyll fluorescence continuance in the process of etiolated leaves greening. Biophysica 7: 571–577 [in Russian]
Seely GR (1978) The energetics of electron-transfer reactions of chlorophyll and other compounds. Photochem Photobiol 27: 639–654
Smith JHC (1952) Factors effecting the transformation of protochlorophyll to chlorophyll. Carnegie Inst Wash Year Book 51: 151–153
Townley HE, Griffith WT and Nugent JP (1998) A reappraisal of the mechanism of the photoenzyme protochlorophyllide reductase based on studies with the heterologously expressed protein. FEBS Lett 422: 19–22
van Bochove AC, Griffiths WT and van Grondelle R (1984) The primary reaction in the photoreduction of protochlorophyllide monitored by nanosecond fluorescence measurements. Photochem Photobiol 39: 101–106
Walker CJ and Griffith WT (1988) Protochlorophyllide reductase: a flavoprotein? FEBS Lett 239: 259–262
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raskin, V.I., Schwartz, A. The charge-transfer complex between protochlorophyllide and NADPH: an intermediate in protochlorophyllide photoreduction. Photosynthesis Research 74, 181–186 (2002). https://doi.org/10.1023/A:1020955526882
Issue Date:
DOI: https://doi.org/10.1023/A:1020955526882