Abstract
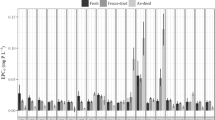
The impact of sediment coring on measured rates of sulfate reduction(SRR) by the whole core 35S-injection technique was assessed inmarshsediment vegetated by Spartina anglica. Simultaneously,therole of extraction method (centrifugation vs. sippers) for determination ofporewater DOC in vegetated sediment was evaluated. SRR was measuredinsitu with radiotracer injected directly into the sediment and in atime series from 1 to 24 h after coring. SRR incubations carriedout within 6 h (June) or 12 h (August) of coringyielded up to an order of magnitude higher rates than measured insitu. The enhancement of SRR was instantaneous but temporary andcorrelated with measured porewater DOC concentrations. Cores sampled fromrootedsediments should therefore not be used for sulfate reduction incubations withinthe first 12 h due to the effect of DOC leaching from roots cutduring the coring procedure. The labile fraction of leached DOC appears to beexhausted after a pre-incubation period of at least 12 h.Measurement of porewater DOC is also problematic in vegetated sediment.Porewater extraction by centrifugation of sediment may result in up to oneorderof magnitude higher DOC concentrations than in porewater obtained by anondestructive sipper technique. DOC is probably forced out of roots duringcentrifugation resulting in erroneously high porewater DOC concentrations.
Similar content being viewed by others
References
Alongi D.M. 1998. Coastal Ecosystem Processes. CRCPress, London.
Blaabjerg V., Mouritsen K.N. and Finster K. 1998. Diel cycles of sulphate reduction rates in sediments of a Zosters marina bed (Denmark). Aquatic Microbial Ecology 15: 97–102.
Fossing H. and Jørgensen B.B. 1989. Measurement of bacterial sulfate reduction in sediments: Evaluation of a single-step chromium reduction method. Biogeochemistry 8: 205–222.
Hall P. and Aller R.C. 1992. Rapid small-volume flow injection 1 analysis for ΣCO2 and \({\text{NH}}_{\text{4}}^{\text{ + }} \) in marine and freshwaters. Limnology and Oceanography 37: 1113–1119.
Hansen J.W. and Lomstein B.A. 1999. Leakage of ammonium, urea, and dissolved organic nitrogen and carbon from eelgrass Zostera marina roots and rhizomes during sediment handling. Aquatic Microbial Ecology 16: 303–307.
Hemminga M.A., Huiskes A.H.L., Steegstra M. and van Soelen J. 1996. Assessment of carbon allocation and biomass production in a natural stand of the salt marsh plant Spartina anglica using 13C. Marine Ecology Progress Series 130: 169–178.
Hines M.E., Knollmeye S.L. and Tugel J.B. 1989. Sulfate reduction and other sedimentary biogeochemistry in a northern New England salt marsh. Limnology and Oceanography 34: 578–590.
Hines M.E., Banta G., Giblin A. and Hobbie J.E. 1994. Acetate concentrations and oxidation in salt-marsh sediments. Limnology and Oceanography 39: 140–148.
Hines M.E., Evans R.S., Genthner B.R.S., Willis S.G., Friedman S., Rooney-Varga J.N. et al. 1999. Molecular Phylogenetic and Biogeochemical Studies of Sulfate-Reducing Bacteria in the Rhizosphere of Spartina alterniflora. Applied and Environmental Microbiology 65: 2209–2216.
Holmer M. and Nielsen S.L. 1997. Sediment sulfur dynamics related to biomass-density pattern in Zostera marina (eelgrass) beds. Marine Ecology Progress Series 146: 163–171.
Holmer M., Jensen H.S., Christensen K.K., Wigand C. and Andersen F.Ø. 1998. Sulfate reduction in lake sediments inhabited by the isoetid macrophytes Littorella uniflora and Isoetes lacustris. Aquatic Botany 60: 307–324.
Holmer M., Gribsholt B. and Kristensen K. 2002. Effects of sea level rise on growth of Spartina anglica and oxygen dynamics in rhizosphere and salt marsh sediments. Marine Ecology Progress Series 225: 197–204.
Howarth R.W. and Hobbie J.E. 1982. The regulation of decomposition and heterotrophic activity in salt marsh soils: A review. In: Kennedy V.S.(ed.), Estuarine Comparisons. Academic Press, NY, USA.
Howarth R.W. 1993. Microbial processes in salt-marsh sediments. In: Ford T.E. (ed.), Aquatic Microbiology. An ecological approach. Blackwell Scientific Publications, Boston, pp. 239–259.
Howes B.L., Dacey J.W.H. and Wakeham S.G. 1985. Effects of sampling technique on measurements of porewater constituents in salt marsh sediments. Limnology and Oceanography 30: 221–227.
Jørgensen B.B. 1978. A comparison of methods for quantification of bacterial sulfate reduction in coastal marine sediments. Geomicrobiology Journal 1: 11–27.
King G.M. 1988. Patterns of sulfate reduction and the sulfur cycle in a South Carolina salt marsh. Limnology and Oceanography 33: 376–390.
Kristensen E. and Andersen F.Ø. 1987. Determination of organic carbon in marine sediments: a comparison of two CHN-analyzer methods. Journal of Experimental Marine Biology and Ecology 109: 15–23.
Mendelssohn I.A., McKee K.L. and Patrick W.H. Jr 1981. Oxygen deficiency in Spartina alterniflora roots: Metabolic adaptation to anoxia. Science 214: 439–411.
Moriarty D.J.W., Iversen R.L. and Pollard P.C. 1986. Exudation of organic carbon by the seagrass Holodule wirghtii Aschers. and its effect on bacterial growth in the sediment. Journal of Ex perimental Marine Biology and Ecology 96: 115–126.
Nedwell B.D., Blackburn T.H. and Wiebe W.J. 1994. Dynamic nature of the turnover of organic carbon, nitrogen and sulphur in the sediment of a Jamaican mangrove forest. Marine Ecology Progress Series 110: 223–231.
Smith R.D., Pregnall A.M. and Alberte R.S. 1984. Effects of anaerobiosis on root metabolism of Zostera marina (eelgrass): implications for survival in reducing sediments. Marine Biology 98: 131–141.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gribsholt, B., Kristensen, E. Impact of sampling methods on sulfate reduction rates and dissolved organic carbon (DOC) concentrations in vegetated salt marsh sediments. Wetlands Ecology and Management 10, 371–379 (2002). https://doi.org/10.1023/A:1020940314010
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1020940314010