Abstract
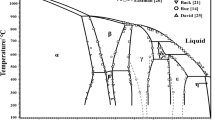
The Bi2O3–SnO2 system was studied by differential thermal analysis, x-ray diffraction, and Knudsen cell mass spectrometric measurements. Bi2Sn2O7 was shown to have an orthorhombic structure with lattice parameters a= 12.262 Å, b = 3.765 Å, and c = 7.957 Å. Mass spectrometry was used to determine the vapor composition over the condensed phase in different phase regions and the partial pressures of the main vapor species. The standard enthalpy of formation of Bi2Sn2O7 was calculated. Based on the experimental data, the T–x and p–T projections of the Bi2O3–SnO2 phase diagram were mapped out.
Similar content being viewed by others
REFERENCES
Muraoka, M., Suzuki, M., Sawada, Y., and Matsushita, J., Sintering of Tin-Doped Indium Oxide (Indium–Tin– Oxide, ITO) with Bi2O3 Additive, J. Mater. Sci., 1998, vol. 33, pp. 5621–5624.
Wang, C.C., Akbar, S.A., and Madou, M.J., Ceramic Based Resistive Sensors, J. Electroceram., 1998, vol. 2, no. 4, pp. 273–282.
Vicent, F., Morallon, E., Quijada, C., et al., Characterization and Stability of Doped SnO2 Anodes, J. Appl. Elecrochem., 1998, vol. 28, pp. 607–612.
Diagrammy sostoyaniya sistem tugoplavkikh oksidov: Spravochnik (Phase Diagrams of Refractory Oxide Systems: A Handbook), issue 5: Dvoinye sistemy (Binary Systems), Galakhov, F.Ya., Ed., Leningrad: Nauka, 1986, part 2, pp. 298–299.
Kargin, Yu.F., Nelyapina, N.I., and Skorikov, V.M., System Bi2O3–SnO2, in Fiziko-khimicheskie issledovaniya ravnovesii v rastvorakh (Physicochemical Studies of Equilibria in Solutions), Yaroslavl: Yaroslav. Gos. Pedagogicheskii Univ. im. K.D. Ushinskogo, 1986, pp. 81–83.
Shannon, R.D., Beirlein, J.D., Gillson, J.L., et al., Polymorphism in Bi2Sn2O7, J. Phys. Chem. Solids, 1980, vol. 41, pp. 117–122.
Roth, R.S., Pyrochlore-Type Compounds Containing Double Oxides of Trivalent and Tetravalent Ions, J. Res. Natl. Bur. Stand., 1956, vol. 56, pp. 17–25.
Levin, E.M. and Roth, R.S., Polymorphism of Bismuth Sesquioxide: II. Effect of Oxide Additions on the Polymorphism of Bi2O3, J. Res. Natl. Bur. Stand., Sect. A, 1964, vol. 68, pp. 197–206.
Vetter, G., Queyroux, F., and Gilles, J.-C., Préparation, stabilité et étude cristallographique préliminaire du composé Bi2Sn2O7, Mater. Res. Bull., 1978, vol. 13, pp. 211-216.
Brisse, F. and Knop, O., Pyrochlores: III. X-ray, Neutron, Infrared, and Dielectric Studies of A2Sn2O7 Stannates, Can. J. Chem., 1968, vol. 46, pp. 859–873.
Gattow, G. and Fricke, H., Beitrag zu den binaren Systemen des Bi2O3 mit SiO2, GeO2 und SnO2, Z. Anorg. Allg. Chem., 1963, vol. 324, pp. 287–296.
Grigor'ev, I.S. and Meilikhov, E.Z., Fizicheskie velichiny: Spravochnik (Physical Quantities: A Handbook), Moscow: Energoatomizdat, 1991, p. 291.
Kazenas, E.K. and Tsvetkov, Yu.V., Isparenie oksidov (Vaporization of Oxides), Moscow: Nauka, 1997.
Kireev, V.A., Metody prakticheskikh raschetov v termodinamike khimicheskikh reaktsii (Practical Calculations in Thermodynamics of Chemical Reactions), Moscow: Khimiya, 1975.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Asryan, N.A., Kol'tsova, T.N., Alikhanyan, A.S. et al. Thermodynamics and Phase Diagram of the Bi2O3–SnO2 System. Inorganic Materials 38, 1141–1147 (2002). https://doi.org/10.1023/A:1020918616870
Issue Date:
DOI: https://doi.org/10.1023/A:1020918616870