Abstract
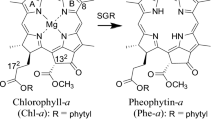
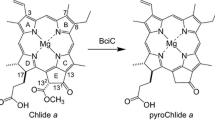
The Mg-dechelating activity of extracts of Chenopodium album (goosefoot) was investigated using an artificial substrate, chlorophyllin a. The activity was measured spectrophotometrically by the formation of a reaction product, pheophorbin a (Mg-free chlorin), after release of the central Mg. The Mg-releasing protein was highly purified by successive DEAE, Butyl and HW-55 chromatographies. The molecular weight of the purified protein was 20 k by gel filtration. The protein showed a broad, but single, pH optimum at 7.5. The K m value for chlorophyllin a was 95.1 nM at pH 7.5. The Mg-releasing protein was not active with chlorophyllide a, a native substrate, although it was active with Zn-chlorophyllin a. Similar results were obtained from horseradish peroxidase. Only a small molecular weight, metal-chelating substance (MCS) had Mg-dechelating activity for the native substrate. An inhibitor study showed involvement of radicals in the Mg-dechelation of the Mg-releasing protein. The purified Mg-releasing protein showed neither peroxidase activity nor absorption bands in the visible region, and this indicates that the Mg-releasing protein is clearly distinct from horseradish peroxidase, which is a heme-containing protein. A likely conclusion is that the Mg-releasing protein and horseradish peroxidase are not involved in the Mg-dechelation in the degradation pathway of chlorophylls. The relevance of the participation of MCS in Mg-dechelation in the breakdown of chlorophylls (Chls) is also discussed.
Similar content being viewed by others
References
Azuma R, Takahashi Y, Kurata H, Kawano T, Shimokawa K and Adachi M (1999) Does peroxidase act as a 'Mg-dechelatase'? Plant Peroxidase Newslett 13: 145–151
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Matile P, Hörtensteiner S and Thomas H (1999) Chlorophyll degradation. Annu Rev Plant Physiol Plant Mol Biol 50: 67–95
Owens TG and Falkowski PG (1982) Enzymatic degradation of chlorophyll a by marine phytoplankton in vitro. Phytochemistry 21: 979–984
Schoch S, Helfrich M, Wiktorsson B, Sundqvist C, Rüdiger W and Ryberg M (1995) Photoreduction of zinc protochlorophyllide b with NADPH-protochlorophyllide oxidoreductase from etiolated wheat (Triticum aestivum L.). Eur J Biochem 229: 291–298
Shimokawa K, Hashizume A and Shioi Y (1990) Pyropheophorbide a, a catabolite of ethylene-induced chlorophyll a degradation. Phytochemistry 29: 2105–2106
Shioi Y, Tatsumi Y and Shimokawa K (1991) Enzymatic degradation of chlorophyll in Chenopodium album. Plant Cell Physiol 32: 87–93
Shioi Y, Masuda K and Takamiya K, Shimokawa K (1995) Breakdown of chlorophylls by soluble proteins extracted from leaves of Chenopodium album. J Plant Physiol 145: 416–421
Shioi Y, Tomita N, Tsuchiya T and Takamiya K (1996) Conversion of chlorophyllide to pheophorbide by Mg-dechelating substance in extracts of Chenopodium album. Plant Physiol Biochem 34: 41–47
Suzuki T and Shioi Y (2001) Degradation of chlorophylls: purification and properties of a Mg-releasing protein from Chenopodium album. PS2001 Proceedings (12th International Congress of Photosynthesis), S2–022. CSIRO Publishing, Collingwood, Australia
Takamiya K, Tsuchiya T and Ohta H (2000) Degradation pathway(s) of chlorophyll: what has gene cloning revealed? Trends Plant Sci 5: 426–431
Tsuchiya T, Ohta H, Okawa K, Masuda T, Mikami B, Kita N, Shioi Y and Takamiya K (1997) Purification and characterization of two isozymes of chlorophyllase from mature leaves of Chenopodium album. Plant Cell Physiol 38: 1026–1031
Vicentini F, Iten F and Matile P (1995) Development of an assay for Mg-dechelatase of oilseed rape cotyledons, using chlorophyllin as the substrate. Physiol Plant 94: 57–63
Ziegler R, Blaheta A, Guha N and Schönegge B (1988) Enzymatic formation of pheophorbide and pyropheophorbide during chlorophyll degradation in amutant of Chlorella fusca Shihira et Kraus. J Plant Physiol 132: 327–332
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, T., Shioi, Y. Re-examination of Mg-dechelation reaction in the degradation of chlorophylls using chlorophyllin a as a substrate. Photosynthesis Research 74, 217–223 (2002). https://doi.org/10.1023/A:1020915812770
Issue Date:
DOI: https://doi.org/10.1023/A:1020915812770