Abstract
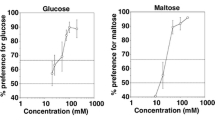
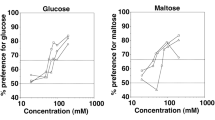
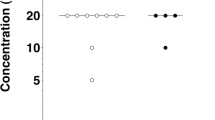
The taste responses to sweet and astringent compounds were investigated in two mammals of similar ecology, by using the classical method of the two-bottle test. The taste threshold for fructose was higher in Microcebus murinus, a prosimian primate, than in Caluromys philander, a didelphid marsupial. The profiles of suprathreshold responses resembled a dissymmetric bell-shaped curve, but the rate of consumption of sweet solutions up to maximal intake increased more rapidly in Microcebus than in Caluromys. Despite showing a photoperiod-synchronized physiology, Microcebus displayed no seasonal variation of the taste threshold and suprathreshold responses. The depressing effect of tanning acid on the ingestion of fructose solutions increased progressively with tannin concentration and was lower as fructose concentration increased. Inhibition thresholds for tannic acid were similar between the two species. The data suggest that adaptation to frugivorous diets is associated with globally similar shaping of the taste responses, even though subtle differences of palatability may account for differences of feeding selectivity.
Similar content being viewed by others
REFERENCES
Atramentowicz, M. 1988. La frugivorie opportuniste de trois Marsupiaux Didelphidés de Guyane. Rev. Ecol. (Terre Vie) 43:47-57.
Barton, R. A. 1992. Allometry of food intake in free-ranging anthropoid primates. Folia Primatol. 58:56-59.
Barton, R. A., Whiten, A., Byrne, R. W., and English, M. 1993. Chemical composition of baboon plant foods: Implications for the interpretation of intra-and interspecific differences in diet. Folia Primatol. 61:1-20.
Charles-Dominique, P. 1983. Ecology and social adaptations in didelphid marsupials: Comparison with eutherians of similar ecology, pp. 395-422, in J. F. Eisenberg and D. G. Kleiman (eds.). Advances in the Study of Mammalian Behavior. Special Publication of the American Society of Mammalogists, Washington, D.C.
Critchley, H., and Rolls, E. 1996. Responses of primate taste cortex neurons to the astringent tastant tannic acid. Chem. Senses 21:135-145.
Feeny, P. 1970. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565-581.
Furstenburg, D., and van Hoven, W. 1994. Condensed tannin as anti-defoliate agent against browsing by giraffe (Giraffa camelopardalis) in the Kruger National Park. Comp. Biochem. Physiol. 170A:425-431.
Ganzhorn, J. U. 1988. Food partitioning among Malagasy primates. Oecologia 75:436-450.
Glaser, D. 1979. Gustatory preference behaviour in primates, pp. 51-61, in J. H. A. Kroeze (ed.). Preference Behaviour and Chemoreception. Information Retrieval Ltd., London.
Glaser, D. 1986. Geschmacksforschung bei Primaten. Vierteljahrschr. Naturforsch. Ges. Zurich 131(2):92-110.
Glaser, D., and Hellekant, G. 1977. Verhaltens-und electrophysiologische Experimente über den Geschmackssinn bei Saguinus midas tamarin (Callitrichidae). Folia Primatol. 28:43-51.
Goldstein, J. L., and Swain, T. 1963. Changes in tannins in ripening fruits. Phytochemistry 2:371-383.
Hellekant, G., Hladik, C. M., Dennys, V., Simmen, B., Roberts, T. W., Glaser, D., DuBois, G., and Walters, D. E. 1993a. On the sense of taste in two Malagasy primates (Microcebus murinus and Eulemur mongoz). Chem. Senses 18:307-320.
Hellekant, G., Hladik, C. M., Dennys, V., Simmen, B., Roberts, T. W., and Glaser, D. 1993b. On the relationship between sweet taste and seasonal body weight changes in a primate (Microcebus murinus). Chem. Senses 18:27-33.
Kawamura, Y., Funakoshi, M., Kasahara, Y., and Yamamoto, T. 1969. A neurophysiological study on astringent taste. Jpn. J. Physiol. 19:851-865.
Kosar, E., and Schwartz, G. 1990. Cortical unit responses to chemical stimulation of the oral cavity in the rat. Brain Res. 513:212-224.
Lebreton, P. 1982. Tannins ou alcaloïdes: deux tactiques phytochimiques de dissuasion des herbivores. Rev. Ecol. (Terre Vie) 36:539-572.
Le Magnen, J. 1987. Central processing of sensory information in the control of feeding, pp. 95-128, in D. Otosson (ed.). Progress in Sensory Physiology. Springer-Verlag, Berlin.
Lindroth, R. L., and Batzli, G. O. 1984. Plant phenolics as chemical defenses: effects of natural phenolics on survival and growth of prairie voles (Microtus ochrogaster). J. Chem. Ecol. 10:229-244.
Lucas, F., and Bellisle, F. 1987. The measurement of food preferences in humans: do taste-and-spit tests predict consumption? Physiol. Behav. 39:739-743.
Lyman, B., and Green, B. 1990. Oral astringency: Effects of repeated exposure and interactions with sweeteners. Chem. Senses 15:151-164.
Marks, D. L., Swain, T., Goldstein, S., Richard, A., and Leighton, M. 1988. Chemical correlates of rhesus monkey food choice: The influence of hydrolyzable tannins. J. Chem. Ecol. 14:213-235.
Michels, R. R., King, J. E., and Hsiao, S. 1988. Preference differences for sucrose solutions in young and aged squirrel monkeys. Physiol. Behav. 42:53-57.
Moskowitz, H. R., Kluter, R. A., Westerling, J., and Jacobs, H. L. 1974. Sugar sweetness and pleasantness: Evidence for different psychological laws. Science 184:583-585.
Ogawa, H., Yamashita, S., Noma, A., and Sato, M. 1972. Taste responses in the macaque monkey chorda tympani. Physiol. Behav. 9:325-331.
Perret, M. 1979. Seasonal and social determinants of urinary catecholamines in the lesser mouse lemur (Microcebus murinus, Cheirogaleinae, Primates). Comp. Biochem. Physiol. 62:51-60.
Perret, M. 1980. Influence de la Captivité et du Groupement Social sur la Physiologie du Microcèbe (Microcebus murinus. Cheirogalinae, Primates). Thèse Doctorat Etat, University Paris XI, Paris.
Perret, M., and Schilling, A. 1987. Intermale sexual effect elicited by volatile urinary ether extract in Microcebus murinus (Prosimian, Primates). J. Chem. Ecol. 13:495-507.
Peter-Rousseaux, A. 1974. Photoperiod, sexual activity and body weight variations of Microcebus murinus (Miller 1777), pp. 365-373, in R. D. Martin and A. C. Walker (eds.). Prosimian Biology. Duckworth, London.
Pfaffmann, C. 1960. The pleasures of sensation. Psychol. Rev. 67:253-268.
Ramirez, I. 1990. Why do sugars taste good? Neurosc. Biobehav. Rev. 14:125-134.
Richter, C. P., and Campbell, K. H. 1939. Sucrose taste thresholds of rats and humans. Am. J. Physiol. 128:291-297.
Robbins, C. T., Hanley, T. A., Hagerman, A. E., Hjeljord, O., Baker, D. L., Schwartz, C. C., and Mautz, W. W. 1987. Role of tannins in defending plants against ruminants: reduction in protein availability. Ecology 68:98-107.
Rolls, E. T., Sienkewicz, Z. J., and Yaxley, S. 1989. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur. J. Neurosci. 1:53-60.
Schiffman, S. S., Suggs, M., Sostman, A., and Simon, S. 1992. Chorda tympani and lingual nerve responses to astringent compounds in rodents. Physiol. Behav. 51:55-63.
Simmen, B. 1994. Taste discrimination and diet differentiation among New World primates, pp. 150-165, in D. J. Chivers and P. Langer (eds.). The Digestive System in Mammals: Food, Form and Function. Cambridge University Press, Cambridge.
Simmen, B., and Hladik, C. M. 1988. Seasonal variation of taste threshold for sucrose in a prosimian species. Microcebus murinus. Folia Primatol. 51:152-157.
Simmen, B., and Hladik, C. M. 1998. Sweet and bitter taste discrimination in primates: scaling effects across species. Folia Primatol. 69:129-138.
Swain, T. 1979. Tanins and lignins, pp. 657-682, in G. A. Rosenthal and D. H. Janzen (eds.). Herbivores, their Interaction with Secondary Plant Metabolites. Academic Press, New York.
Vanderweele, D. A., Novin, D., Rezek, M., and Sanderson, J. D. 1974. Duodenal or hepaticportal glucose perfusion: Evidence for duodenally-based satiety. Physiol. Behav. 12:467-473.
Wrangham, R. W., and Waterman, P. G. 1983. Condensed tannins in fruits eaten by chimpanzees. Biotropica 15:217-222.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Simmen, B., Josseaume, B. & Atramentowicz, M. Frugivory and Taste Responses to Fructose and Tannic Acid in a Prosimian Primate and a Didelphid Marsupial. J Chem Ecol 25, 331–346 (1999). https://doi.org/10.1023/A:1020850914167
Issue Date:
DOI: https://doi.org/10.1023/A:1020850914167