Abstract
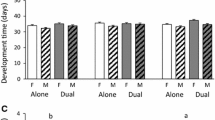
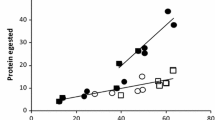
This study explored the effect of resource availability on plant phytochemical composition within the framework of carbon–nutrient balance (CNB) theory. We grew quaking aspen (Populus tremuloides) under two levels of light and three levels of nutrient availability and measured photosynthesis, productivity, and foliar chemistry [water, total nonstructural carbohydrates (TNC), condensed tannins, and phenolic glycosides]. Gypsy moths (Lymantria dispar) and forest tent caterpillars (Malacosoma disstria) were reared on foliage from each of the treatments to determine effects on insect performance. Photosynthetic rates increased under high light, but were not influenced by nutrient availability. Tree growth increased in response to both the direct and interactive effects of light and nutrient availability. Increasing light reduced foliar nitrogen, while increasing nutrient availability increased foliar nitrogen. TNC levels were elevated under high light conditions, but were not influenced by nutrient availability. Starch and condensed tannins responded to changes in resource availability in a manner consistent with CNB theory; levels were highest under conditions where tree growth was limited more than photosynthesis (i.e., high light–low nutrient availability). Concentrations of phenolic glycosides, however, were only moderately influenced by resource availability. In general, insect performance varied relatively little among treatments. Both species performed most poorly on the high light–low nutrient availability treatment. Because phenolic glycosides are the primary factor determining aspen quality for these insects, and because levels of these compounds were minimally affected by the treatments, the limited response of the insects was not surprising. Thus, the ability of CNB theory to accurately predict allocation to defense compounds depends on the response of specific allelochemicals to changes in resource availability. Moreover, whether allelochemicals serve to defend the plant depends on the response of insects to specific allelochemicals. Finally, in contrast to predictions of CNB theory, we found substantial allocation to storage and defense compounds under conditions in which growth was carbon-limited (e.g., low light), suggesting a cost to defense in terms of reduced growth.
Similar content being viewed by others
REFERENCES
Bazzaz, F. A., and Miao, S. L. 1993. Successional status, seed size, and responses of tree seedlings to CO2, light, and nutrients. Ecology 74:104-112.
Bowman, W. D., and Conant, R. T. 1994. Shoot growth dynamics and photosynthetic response to increased nitrogen availability in the alpine willow Salix glauca. Oecologia 97:93-99.
Brown, K., and Higginbotham, K. O. 1986. Effects of carbon dioxide enrichment and nitrogen supply on growth of boreal tree seedlings. Tree Physiol. 2:223-232.
Bryant, J. P. 1987. Feltleaf willow-snowshoe hare interactions: Plant carbon/nutrient balance and floodplain succession. Ecology 68:1319-1327.
Bryant, J. P., Chapin, F. S., III, and Klein, D. R. 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357-368.
Bryant, J. P., Clausen, T. P., Reichardt, P. B., McCarthy, M. C., and Werner, R. A. 1987. Effect of nitrogen fertilization upon the secondary chemistry and nutritional value of quaking aspen (Populus tremuloides Michx.) leaves for the large aspen tortrix [Choristoneura conflictana (Walker)]. Oecologia 73:513-517.
Chapin, F. S., III. 1980. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 11:233-260.
Chapin, F. S., III, Bloom, A. J., Field, C. B., and Waring, R. H. 1987. Plant responses to multiple environmental factors. BioScience 37:49-57.
El Kohen, A., and Mousseau, M. 1994. Interactive effects of elevated CO2 and mineral nutrition on growth and CO2 exchange of sweet chestnut seedlings (Castanea sativa). Tree Physiol. 14:679-690.
Farrar, R. R., Jr., Barbour, J. D., and Kennedy, G. G. 1989. Quantifying food consumption and growth in insects. Ann. Entomol. Soc. Am. 82:593-598.
Hagerman, A. E., and Butler, L. G. 1980. Condensed tannin purification and characterization of tannin-associated proteins. J. Agric. Food. Chem. 28:948-952.
Hartley, S. E., Nelson, K., and Gorman, M. 1995. The effect of fertiliser and shading on plant chemical composition and palatability to Orkney voles, Microtus arvalis orcadensis. Oikos 72:79-87.
Hemming, J. D. C., and Lindroth, R. L. 1995. Intraspecific variation in aspen phytochemistry: Effects on performance of gypsy moths and forest tent caterpillars. Oecologia 103:79-88.
Herms, D. A., and Mattson, W. J. 1992. The dilemma of plants: To grow or defend. Q. Rev. Biol. 67:282-335.
Hwang, S.-Y., and Lindroth, R. L. 1997. Clonal variation in foliar chemistry of aspen: Effects on gypsy moths and forest tent caterpillars. Oecologia 111:99-108.
Kinney, K. K., and Lindroth, R. L. 1997. Responses of three deciduous tree species to atmospheric CO2 and soil NO −3 availability. Can. J. For. Res. 27:1-10.
Kinney, K. K., Lindroth, R. L., Jung, S. M., and Nordheim, E. V. 1997. Effects of atmospheric CO2 and soil NO −3 availability on deciduous trees: phytochemistry and insect performance. Ecology 78:215-230.
Koricheva, J., Larsson, S., Haukioja, E., and KeinÄnen, M. 1998. Regulation of woody plant secondary metabolism by resource availability: Hypothesis testing by means of meta-analysis. Oikos 83:212-226.
Lang, C. A. 1958. Simple microdetermination of Kjeldahl nitrogen in biological materials. Anal. Chem. 30:1692-1694.
Lawler, I. R., Foley, W. J., Woodrow, I. E., and Cork, S. J. 1997. The effects of elevated CO2 atmospheres on the nutritional quality of Eucalyptus foliage and its interaction with soil nutrient and light availability. Oecologia 109:59-68.
Lindroth, R. L., and Hwang, S.-Y. 1996. Clonal variation in foliar chemistry of quaking aspen (Populus tremuloides Michx.). Biochem. Syst. Ecol. 24:357-364.
Lindroth, R. L., Hsia, M. T. S., and Scriber, J. M. 1987. Seasonal patterns in the phytochemistry of three Populus species. Biochem. Syst. Ecol. 15:681-686.
Lindroth, R. L., Kinney, K. K., and Platz, C. L. 1993. Responses of deciduous trees to elevated atmospheric CO2: Productivity, phytochemistry, and insect performance. Ecology 74:763-777.
Littell, R. C., Milliken, G. A., Stroup, W. W., and Wolfinger, R. D. 1996. SAS Systems for Mixed Models. SAS Institute Inc., Cary, North Carolina.
Matsuki, M. 1996. Regulation of plant phenolic synthesis: from biochemistry to ecology and evolution. Aust. J. Bot. 44:613-634.
Mattson, M. J., Jr. 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 11:119-161.
McDonald, A. J. S., Lohammar, T., and Ericsson, A. 1986. Growth response to step-decrease in nutrient availability in small birch (Betula pendula Roth). Plant Cell Environ. 9:427-432.
McDonald, E. P., and Lindroth, R. L. 1999. CO2 and light effects on deciduous trees: Growth, foliar chemistry, and insect performance. Oecologia. In press.
McGuire, A. D., Melillo, J. M., and Joyce, L. A. 1995. The role of nitrogen in the response of forest net primary production to elevated atmospheric carbon dioxide. Annu. Rev. Ecol. Syst. 26:473-503.
Mole, S. 1994. Trade-offs and constraints in plant-herbivore defense theory: a life-history perspective. Oikos 71:3-12.
Parkinson, J. A., and Allen, S. E. 1975. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plant Anal. 6:1-11.
Pettersson, R., McDonald, A. J. S., and Stadenberg, I. 1993. Response of small birch plants (Betula pendula Roth.) to elevated CO2 and nitrogen supply. Plant Cell Environ. 16:1115-1121.
Porter, L. J., Hrstich, L. N., and Chan, B. G. 1986. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25:233-230.
Raubenheimer, D., and Simpson, S. J. 1992. Analysis of covariance: An alternative to nutritional indices. Entomol. Exp. Appl. 62:221-231.
Reichardt, P. B., Chapin, F. S., III, Bryant, J. P., Mattes, B. R., and Clausen, T. P. 1991. Carbon/nutrient balance as a predictor of plant defense in Alaskan balsam poplar: Potential importance of metabolite turnover. Oecologia 88:401-406.
Roth, S. K., and Lindroth, R. L. 1995. Elevated atmospheric CO2: Effects on phytochemistry, insect performance and insect-parasitoid interactions. Global Change Biol. 1:173-182.
Roth, S., McDonald, E. P., and Lindroth, R. L. 1997. Atmospheric CO2 and soil water availability: consequences for tree-insect interactions. Can. J. For. Res. 27:1281-1290.
RuohomÄki, K., Chapin, F. S., III, Haukioja, E., Neuvonen, S., and Suomela, J. 1996. Delayed inducible resistance in mountain birch in response to fertilization and shade. Ecology 77:2302-2311.
SAS Institute. 1989. SAS User's Guide: Statistics, version 6 ed. SAS Institute, Cary, North Carolina.
Tuomi, J., Niemela, P., Haukioja, E., SirÉn, S., and Neuvonen, S. 1988. Defensive responses of trees in relation to their carbon/nutrient balance, pp. 57-72, in W. J. Mattson, J. Levieux, and C. Bern-Dagan (eds.). Mechanisms of Woody Plant Defenses Against Insects—Search for Pattern. Springer-Verlag, New York.
Waldbauer, G. P. 1968. The consumption and utilization of food by insects. Adv. Insect Physiol. 5:229-288.
Walters, M. B., and Reich, P. B. 1996. Are shade tolerance, survival, and growth linked? Low light and nitrogen effects on hardwood seedlings. Ecology 77:841-853.
Walters, M. B., Kruger, E. L., and Reich, P. B. 1993. Growth, biomass distribution and CO2 exchange of northern hardwood seedlings in high and low light: Relationships with successional status and shade tolerance. Oecologia 94:7-16.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hemming, J.D.C., Lindroth, R.L. Effects of Light and Nutrient Availability on Aspen: Growth, Phytochemistry, and Insect Performance. J Chem Ecol 25, 1687–1714 (1999). https://doi.org/10.1023/A:1020805420160
Issue Date:
DOI: https://doi.org/10.1023/A:1020805420160