Abstract
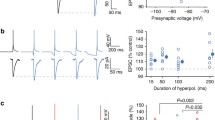
During evoked release, several quanta of neurotransmitter are synchronously released in several GABA-ergic synapses. Assuming that not more than one vesicle is released at each release site, the decay of miniature and evoked IPSC (mIPSC and eIPSC, respectively) should coincide. In this study, we found that in a considerable part of the cultured hippocampal neurons eIPSC decayed more slowly than mIPSC did. We investigated the mechanisms underlying this difference using conventional electrophysiological approaches, deconvolution, simulations, and nonstationary noise analysis. Our results indicate that asynchronous release of synaptic vesicles cannot explain the prolonged decay of the GABA-ergic IPSC. We suggest that some interaction between the quanta at the pre- and/or post-synaptic level should result in a slower decay of the eIPSC in comparison with that of mIPSC.
Similar content being viewed by others
REFERENCES
J. S. Diamond and C. E. Jahr, “Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC,” Neuron, 15,No. 5, 1097–1107 (1995).
R. Schneggenburger and E. Neher, “Intracellular calcium dependence of transmitter release rates at a fast central synapse,” Nature, 406,No. 6798, 889–893 (2000).
P. P. Atluri and W. G. Regehr, “Delayed release of neurotransmitter from cerebellar granule cells,” J. Neurosci., 18,No. 20, 8214–8227 (1998).
A. G. Carter and W. G. Regehr, “Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse,” J. Neurosci., 20,No. 12, 4423–4434 (2000).
D. M. Kullmann and F. Asztely, “Extrasynaptic glutamate spillover in the hippocampus: evidence and implications,” Trends Neurosci., 21,No. 1, 8–14 (1998).
I. V. Melnick, M. A. Chvanov, and P. V. Belan, “Rat hippocampal neurons maintain their own GABAergic synaptic transmission in culture,” Neurosci. Lett. 262,No. 3, 151–154 (1999).
S. F. Traynelis, “Software-based correction of single compartment series resistance errors,” J. Neurosci. Methods, 86,No. 1, 25–34 (1998).
E. Neher and T. Sakaba, “Combining deconvolution and noise analysis for the estimation of transmitter release rates at the calyx of held,” J. Neurosci., 21,No. 2, 444–461 (2001).
J. R. Segal, B. Ceccarelli, R. Fesce, and W. P. Hurlbut, “Miniature endplate potential frequency and amplitude determined by an extension of Campbell's theorem,” Biophys. J., 47,No. 2, Part 1, 183–202 (1985).
M. L. Hines and N. T. Carnevale, “The NEURON simulation environment,” Neural Comput., 9,No. 6, 1179–1209 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stepanyuk, A., Chvanov, M., Ivanov, A. et al. Prolonged Decay of Evoked Inhibitory Postsynaptic Currents in Hippocampal Neurons Is Not Shaped by Asynchronous Release. Neurophysiology 34, 239–242 (2002). https://doi.org/10.1023/A:1020792409135
Issue Date:
DOI: https://doi.org/10.1023/A:1020792409135