Abstract
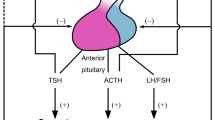
Biological activities of the multifunctional cytokine, interleukin-6 (IL-6) include stimulation of B cell proliferation, immunoglobulin production, and initiation of the acute-phase response. IL-6 affects the CNS in that it activates the hypothalamo-pituitary-adrenocortical (HPA) axis and increases brain tryptophan and serotonin metabolism. IL-6 has been proposed as an important mediator of interaction between the neuroendocrine and immune systems. The peripheral and central effects of IL-6 are presumably mediated through its membrane receptor (IL-6R). IL-6, IL-6R and their respective mRNAs have been detected in several brain regions. Although the functions of cytokines overlap considerably, each displays its own characteristic properties. Expression of IL-6 in the brain has been observed in several CNS disorders, some of which have been associated with disorders of serotonin metabolism. It is proposed that interactions between IL-6 and brain serotonin is a complex process which involves corticotropin-releasing factor (CRF) and opioid peptides. It is likely that the molecular mechanisms underlying the actions of IL-6 on the HPA axis and its other brain functions involve the integrated effects of glutamate, Ca2+, 3′,5′-cyclic AMP, protein kinase C, and other metabolic pathways.
Similar content being viewed by others
REFERENCES
Akira, S., Taga, T., and Kishimoto, T., 1993. Interleukin-6 in biology and medicine. Adv. Immunol. 54:1-78.
Kishimoto, T., Akira, S., and Taga, T., 1992. Interleukin-6 and its receptor: a paradigm for cytokines. Science 258:593-597.
Barton, B. E., 1996. The biological effects of interleukin-6. Med. Res. Rev. 16:87-109.
Van Snick, J., 1990. Interleukin-6: an overview. Annu. Rev. Immunol. 8:253-278.
Baumann, H., and Gauldie, J., 1994. The acute phase response. Immunol. Today 15:74-80.
Castell, J. V., Gómez-Lechón, M. J., David, M., Andus, T., Geiger, T., Trullenque, R., Fabra, R., and Heinrich, P. C., 1989. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Letts 242:237-239.
Opp, M., Obal, F., Cady, A. B., Johannsen, L., and Krueger, J. M., 1989. Interleukin-6 is pyrogenic but not somnogenic. Physiol. Behav. 45:1069-1072.
LeMay, L. G., Vander, A. J., and Kluger, M. J., 1990. Role of interleukin 6 in fever in rats. Amer. J. Physiol. 258:R798-803.
Rothwell, N. J., Busbridge, N. J., Lefeuvre, R. A., Hardwick, A. J., Gauldie, J., and Hopkins, S. J., 1991. Interleukin-6 is a centrally acting endogenous pyrogen in the rat. Can. J. Physiol. Pharmacol. 69:1465-1469.
Klir, J. J., Roth, J., Szelényi, Z., McClellan, J. L., and Kluger, M. J., 1993. Role of hypothalamic interleukin-6 and tumor necrosis factor-α in LPS fever in rat. Amer. J. Physiol. 265:R512-517.
Wang, J. P., Ando, T., and Dunn, A. J., 1997. Effect of homologous interleukin-1, interleukin-6 and tumor necrosis factor α on the core body temperature of mice. NeuroImmunomodulation 4:230-286.
del Rey, A., and Besedovsky, H. O., 1992. Metabolic and neuroendocrine effects of pro-inflammatory cytokines. Europ. J. Clin. Invest. 22:10-15.
Lyson, K., and McCann, S. M., 1992. Involvement of arachidonic acid cascade pathways in interleukin-6-stimulated corticotropin-releasing factor release in vitro. Neuroendocrinol. 55:708-713.
Matta, S. G., Weatherbee, J., and Sharp, B. M., 1992. A central mechanism is involved in the secretion of ACTH in response to IL-6 in rats: comparison to and interaction with IL-1β. Neuroendocrinol. 56:516-525.
Wang, J. P., and Dunn, A. J., 1998. Mouse interleukin-6 stimulates the HPA axis and increases brain tryptophan and serotonin metabolism. Neurochem. Intl 33:143-154.
Dunn, A. J., 1995. Interactions between the nervous system and the immune system: implications for psychopharmacology, Pages 719-731, in Bloom, F. E., and Kupfer, D. J. (eds.), Psychopharmacology: The Fourth Generation of Progress, Raven Press, New York.
Besedovsky, H. O., and del Rey, A., 1996. Immune-neuroendocrine interactions: facts and hypotheses. Endocrine Rev. 17:64-102.
Jones, T. H., 1994. Interleukin-6 an endocrine cytokine. Clin. Endocrinol. 40:703-713.
Plata-Salamán, C. R., 1991. Immunoregulators in the nervous system. Neurosci. Biobehav. Rev. 15:185-215.
Hopkins, S. J., and Rothwell, N. J., 1995. Cytokines and the nervous system I: expression and recognition. TINS 18:83-88.
Schöbitz, B., de Kloet, E. R., and Holsboer, F., 1994. Gene expression and function of interleukin-1, interleukin-6 and tumor necrosis factor in the brain. Prog. Neurobiol. 44:397-432.
Besedovsky, H. O., and Del Rey, A., 1992. Immune-neuroendocrine circuits: integrative role of cytokines. Front. Neuroendocrinol. 13:61-94.
Lieberman, A. P., Pitha, P. M., Shin, H. S., and Shin, M. L., 1989. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc. Natl. Acad. Sci. USA. 86:6348-6352.
Norris, J. G., Tang, L. P., Sparacio, S. M., and Benveniste, E. N., 1994. Signal transduction pathways mediating astrocyte IL-6 induction by IL-1 beta and tumor necrosis factor-alpha. J. Immunol. 152:841-850.
Lieb, K., Kaltschmidt, C., Kaltschmidt, B., Baeuerle, P. A., Berger, M., Bauer, J., and Fiebich, B. L., 1996. Interleukin-1β uses common and distinct signaling pathways for induction of the interleukin-6 and tumor necrosis factor α genes in the human astrocytoma cell line U373. J. Neurochem. 66:1496-1503.
Lafortune, L., Nalbantoglu, J., and Antel, J. P., 1996. Expression of tumor necrosis factor alpha (TNF alpha) and interleukin 6 (IL-6) mRNA in adult human astrocytes: comparison with adult microglia and fetal astrocytes. J. Neuropathol. Exp. Neurol. 55:515-521.
Sébire, G., Emilie, D., Wallon, C., Héry, C., Devergne, O., Delfraissy, J.-F., Galanaud, P., and Tardieu, M., 1993. In vitro production of IL-6, IL-1β, and tumor necrosis factor-α by human embryonic microglial and neural cells. J. Immunol. 150:1517-1523.
Yamasaki, K., Taga, T., Hirata, Y., Yawata, H., Kawanishi, Y., Seed, B., Taniguchi, T., Hirano, T., and Kishimoto, T., 1988. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science 241:825-828.
Murakami, M., Hibi, M., Nakagawa, N., Nakagawa, T., Yasukawa, K., Yamanishi, K., Taga, T., and Kishimoto, T., 1993. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 260:1808-1810.
Narazaki, M., Yasukawa, K., Saito, T., Ohsugi, Y., Fukui, H., Koishihara, Y., Yancopoulos, G. D., Taga, T., and Kishimoto, T., 1993. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood 82:1120-1126.
Suzuki, H., Yasukawa, K., Saito, T., Narazaki, M., Hasegawa, A., Taga, T., and Kishimoto, T., 1993. Serum soluble IL-6 receptor in MRL/1 pr mice is elevated with age and mediates the IL-6 signal. Europ. J. Immunol. 23:1078-1082.
Gadient, R. A., and Otten, U., 1993. Differential expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) messenger RNAs in rat hypothalamus. Neurosci. Letts 153:13-16.
Schöbitz, B., de Kloet, E. R., Sutanto, W., and Holsboer, F., 1993. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Europ. J. Neurosci. 5:1426-1435.
Schöbitz, B., Voorhuis, D. A. M., and De Kloet, E. R., 1992. Localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Neurosci. Letts 136:189-192.
Hama, T., Kushima, Y., Mityamoto, M., Kubota, M., Takei, N., and Hatanaka, H., 1991. Interleukin-6 improves the survival of mesencephalic catecholaminergic and septal cholinergic neurons from postnatal two-week old rats in cultures. Neurosci. 40:445-452.
Otten, U., Heese, K., März, P., and Rose-John, S., 1998. Region-specific neurotrophin expression by interleukin-6-activated rat astrocytes. Soc. Neurosci. Abstr. 24:1288.
Fiebich, B. L., Biber, K., Gyufko, K., Berger, M., Bauer, J., and van Calker, D., 1996. Adenosine A2b receptors mediate an increase in interleukin (IL)-6 mRNA and IL-6 protein synthesis in human astroglioma cells. J. Neurochem. 66:1426-1431.
Kozawa, O., Suzu, A., Tokuda, H., Kaida, T., and Uematsu, T., 1998. Interleukin-6 synthesis induced by prostaglandin E2: cross-talk regulation by protein kinase C. Bone 22:355-360.
Fiebich, B. L., Lieb, K., Berger, M., and Bauer, J., 1995. Stimulation of the sphingomyelin pathway induces interleukin-6 gene expression in human astrocytoma cell. J. Neuroimmunol. 63:207-211.
Maimone, D., Cioni, C., Rosa, S., Macchia, G., Aloisi, F., and Annunziata, P., 1993. Norepinephrine and vasoactive intestinal peptide induce IL-6 secretion by astrocytes: synergism with IL-1 beta and TNF alpha. J. Neuroimmunol. 47:73-81.
Onyia, J. E., Libermann, T. A., Bidwell, J., Arnold, D., Tu, Y., McClelland, P., and Hock, J. M., 1997. Parathyroid hormone (1–34)-mediated interleukin-6 induction. J. Cell. Biochem. 67:265-274.
Zhang, Y., Lin, J. X., and Vilcek, J., 1988. Synthesis of interleukin 6 (interferon-β2/B cell stimulatory factor 2) in human fibroblasts is triggered by an increase in intracellular cyclic AMP. J. Biol. Chem. 263:6177-7182.
Sehgal, P. B., Walther, Z., and Tamm, I., 1987. Rapid enhancement of β2-interferon/B cell differentiation factor BSF-2 gene expression in human fibroblasts by diacylglycerols and the calcium ionophore A23187. Proc. Natl. Acad. Sci. USA 84:3663-3667.
Sehgal, P. B., 1992. Regulation of IL 6 gene expression. Res. Immunol. 143:724-734.
Dendorfer, U., Oettgen, P., and Libermann, T. A., 1994. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP and lipopolysaccharide. Molec. Cell. Biol. 14:4443-4454.
Kawashima, S., Hayashi, M., Takii, T., Kimura, H., Zhang, H. L., Nagatsu, A., Sakakibara, J., Murata, K., Oomoto, Y., and Onozaki, K., 1998. Serotonin derivative, N-(p-coumaroyl) serotonin, inhibits the production of TNF-alpha, IL-1α, IL-1β, and IL-6 by endotoxin-stimulated human blood monocytes. Intl. Cytokine Res. 18:423-428.
Dunn, A.J., 1992. The role of interleukin-1 and tumor necrosis factor α in the neurochemical and neuroendocrine responses to endotoxin. Brain Res. Bull. 29:807-812.
Zalcman, S., Green-Johnson, J. M., Murray, L., Nance, D. M., Dyck, D., Anisman, H., and Greenberg, A. H., 1994. Cytokine-specific central monoamine alterations induced by interleukin-1,-2 and-6. Brain Res. 643:40-49.
Terao, A., Oikawa, M., and Saito, M., 1994. Tissue-specific increase in norepinephrine turnover by central interleukin-1, but not by interleukin-6, in rats. Amer. J. Physiol. 266:R400-404.
Dunn, A. J., 1992. Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J. Pharmacol. Exptl Therapeut. 261:964-969.
Dunn, A. J., and Welch, J., 1991. Stress-and endotoxin-induced increases in brain tryptophan and serotonin metabolism depend on sympathetic nervous system activation. J. Neurochem. 57:1615-1622.
Heyes, M. P., Quearry, B. J., and Markey, S. P., 1989. Systemic endotoxin increases L-tryptophan, 5-hydroxyindoleacetic acid, 3-hydroxykynurenine and quinolinic acid content of mouse cerebral cortex. Brain Res. 491:173-179.
Masana, M. I., Heyes, M. P., and Mefford, I. N., 1990. Indomethacin prevents increased catecholamine turnover in rat brain following systemic endotoxin challenge. Prog. Neuro-Psychopharmacol. Biol. Psychiat. 14:609-621.
Linthorst, A. C. E., Flackskamm, C., Holsboer, F., and Reul, J. M. H. M., 1996. Activation of serotonergic and noradrenergic neurotransmission in the rat hippocampus after peripheral administration of bacterial endotoxin: involvement of the cyclo-oxygenase pathway. Neuroscience 72:989-997.
Linthorst, A. C. E., Flanchskamm, C., Müller-Preuss, P., Holsboer, F., and Reul, J. M. H. M., 1995. Effect of bacterial endotoxin and interleukin-1β on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: an in vivo microdialysis study. J. Neurosci. 15:2920-2934.
Lavicky, J., and Dunn, A. J., 1995. Endotoxin administration stimulates cerebral catecholamine release in freely moving rats as assessed by microdialysis. J. Neurosci. Res. 40:407-413.
Shintani, F., Kanba, S., Nakaki, T., Nibuya, M., Kinoshita, N., Suzuki, E., Yagi, G., Kato, R., and Asai, M., 1993. Interleukin-1β augments release of norepinephrine, dopamine, and serotonin in the rat anterior hypothalamus. J. Neurosci. 13:3574-3581.
Linthorst, A. C. E., Flachskamm, C., Holsboer, F., and Reul, J. M., 1994. Local administration of recombinant human interleukin-1β in the rat hippocampus increases serotonergic neurotransmission, hypothalamic-pituitary-adrenocortical axis activity and body temperature. Endocrinol. 135:520-532.
Dunn, A. J., Powell, M. L., Meitin, C., and Small, P. A., 1989. Virus infection as a stressor: influenza virus elevates plasma concentrations of corticosterone, and brain concentrations of MHPG and tryptophan. Physiol. Behav. 45:591-594.
Dinarello, C. A., Cannon, J. G., Mancilla, J., Bishai, I., Lees, J., and Coceani, F., 1991. Interleukin-6 as an endogenous pyrogen: induction of prostaglandin E2 in brain but not in peripheral blood mononuclear cells. Brain Res. 562:199-206.
Perlstein, R. S., Whitnall, M. H., Abrams, J. S., Mougey, E. H., and Neta, R., 1993. Synergistic roles of interleukin-6, interleukin-1, and tumor necrosis factor in the adrenocorticotropin response to bacterial lipopolysaccharide in vivo. Endocrinol. 132:946-952.
Wang, J. P., and Dunn, A. J., 1999. The role of interleukin-6 in the activation of the hypothalamo-pituitary-adrenocortical axis induced by endotoxin and interleukin-1β. Brain Res. 815:337-348.
Alper, R. H., 1990. Evidence for central and peripheral serotonergic control of corticosterone secretion in the conscious rats. Neuroendocrinol. 51:255-260.
Bagdy, G., Calogero, A. E., Murphy, D. L., and Szemeredi, K., 1989. Serotonin agonists cause parallel activation of the sympathoadrenomedullary system and the hypothalamo-pituitary-adrenocortical axis in conscious rats. Endocrinol. 125:2664-2669.
Korte, S. M., Van Duin, S., Bouws, G. A. H., Koolhaas, J. M., and Bohus, B., 1991. Involvement of hypothalamic serotonin in activation of the sympathoadrenomedullary system and hypothalamo-pituitary-adrenocortical axis in male Wistar rats. Europ. J. Pharmacol. 197:225-228.
Pan, L., and Gilbert, F., 1992. Activation of 5-HT1A receptor subtype in the paraventricular nuclei of the hypothalamus induces CRH and ACTH release in the rat. Neuroendocrinol. 56:797-802.
Dunn, A. J., and Chuluyan, H., 1992. The role of cyclo-oxygenase and lipoxygenase in the interleukin-1-induced activation of the HPA axis: dependence on the route of injection. Life Sci. 51:219-225.
Dunn, A. J., 1993. Nitric oxide synthase inhibitors prevent the cerebral tryptophan and serotonergic responses to endotoxin and interleukin-1. Neurosci. Res. Commun. 13:149-156.
Dunn, A. J., 1999. Brain catecholaminergic and tryptophan responses to restraint are attenuated by nitric oxide synthase inhibition. Neurochem. Intl 33:551-557.
Vale, W., Spiess, J., Rivier, C., and Rivier, J., 1981. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213:1394-1397.
Saphier, D., and Ovadia, H., 1990. Selective facilitation of putative corticotropin-releasing factor-secreting neurones by interleukin-1. Neurosci. Letts 114:283-288.
Sapolsky, R., Rivier, C., Yamamoto, G., Plotsky, P., and Vale, W., 1987. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 238:522-524.
Uehara, A., Gottschall, P. E., Dahl, R. R., and Arimura, A., 1987. Interleukin-1 stimulates ACTH release by an indirect action which requires endogenous corticotropin releasing factor. Endocrinol. 121:1580-1582.
Dunn, A. J., 1993. Role of cytokines in infection-induced stress. Ann. N.Y. Acad. Sci. 697:189-202.
Berkenbosch, F., van Oers, J., del Rey, A., Tilders, F., and Besedovsky, H., 1987. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science 238:524-526.
Takao, T., Newton, R. C., and De Souza, E. B., 1993. Species differences in [125I]interleukin-1 binding in brain, endocrine and immune tissues. Brain Res. 623:172-176.
Haour, F., Ban, E., Marquette, C., Milon, G., and Fillion, G., 1992. Brain Interleukin-1 receptors: mapping, characterization and modulation, Pages 13-25, in Rothwell, N. J., and Dantzer, R. D. (eds.), Interleukin-1 in the Brain, Pergamon Press, Oxford.
Dunn, A. J., 1988. Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism parallelling the increased plasma corticosterone. Life Sci. 43:429-435.
Kabiersch, A., del Rey, A., Honegger, C. G., and Besedovsky, H. O., 1988. Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain Behav. Immun. 2:267-274.
Chuluyan, H., Saphier, D., Rohn, W. M., and Dunn, A. J., 1992. Noradrenergic innervation of the hypothalamus participates in the adrenocortical responses to interleukin-1. Neuroendocrinol. 56:106-111.
Weidenfeld, J., Abramsky, O., and Ovadia, H., 1989. Evidence for the involvement of the central adrenergic system in interleukin 1-induced adrenocortical response. Neuropharmacol. 28:1411-1414.
Chang, S. L., Ren, T., and Zadina; J. E., 1993. Interleukin-1 activation of Fos protooncogene protein in the rat hypothalamus. Brain Res. 617:123-130.
Day, H. E. W., and Akil, H., 1996. Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology 63:207-218.
Swiergiel, A. H., Dunn, A. J., and Stone, E. A., 1996. The role of cerebral noradrenergic systems in the Fos response to interleukin-1. Brain Res. Bull. 41: 61-64.
Tsigos, C., Papanicolaou, D. A., Defensor, R., Mitsiadis, C. S., Kyrou, I., and Chrousos, G. P., 1997. Dose effects of recombinant human interleukin-6 on pituitary hormone secretion and energy expenditure. Neuroendocrinol. 66:54-62.
Naitoh, Y., Fukata, J., Tominaga, T., Nakai, Y., Tamai, S., Mori, K., and Imura, H., 1988. Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious, freely-moving rats. Biochem. Biophys. Res. Commun. 155:1459-1463.
Lyson, K., and McCann, S. M., 1991. The effect of interleukin-6 on pituitary hormone release in vivo and in vitro. Neuroendocrinol. 54:262-266.
Vallieres, L., and Rivest, S., 1998. Permissive role of interleukin-6 and its receptor on the neural activity and neuroendocrine CRF gene expression during endotoxeamia. Soc. Neurosci. Abstr. 24:1859.
Fuller, R. W., 1990. Serotonin receptors and neuroendocrine responses. Neuropsychopharmacol. 3:495-502.
McElroy, J. F., Miller, J. M., and Meyer, J. S., 1984. Fenfluramine, p-chloroamphetamine and p-fluoroamphetamine stimulation of pituitary adrenocortical activity in the rat: evidence for differences in site and mechanism of action. J. Pharmacol. Exptl. Therap. 228:593-599.
Jones, M. T., Hillhouse, E. W., and Burden, J. L., 1976. Effect on various putative neurotransmitters on the secretion of corticotrophin-releasing hormone from the rat hypothalamus in vitro—a model of the neurotransmitters. J. Endocrinol. 69:1-10.
Gibbs, D. M., and Vale, W., 1983. Effect of the serotonin reuptake inhibitor fluoxetine on corticotropin-releasing factor and vasopressin secretion into hypophysial portal blood. Brain Res. 280:176-179.
Grossman, A., Costa, A., Navarra, P., and Tsagarakis, S., 1993. The regulation of hypothalamic corticotropin-releasing factor release: in vitro studies, Pages 129-150, in Chadwick, D. J., Marsh, J., and Ackrill, K. (eds.), Corticotropin-Releasing Factor, Wiley-Interscience Publication, London.
Le Feuvre, R. A., Aisenthal, L., and Rothwell, N. J., 1991. Involvement of corticotrophin releasing factor (CRF) in the thermogenic and anorexic actions of serotonin (5-HT) and related compounds. Brain Res. 555:245-250.
Samanin, R., and Garattini, S., 1989. Serotonin and the pharmacology of eating disorders. Ann. N.Y. Acad. Sci. 575:194-207.
Rothwell, N. J., and Stock, M. J., 1987. Effect of diet and fenfluramine on thermogenesis in the rat: possible involvement of serotonergic mechanisms. Intl. J. Obes. 11:319-324.
Appel, N. M., Owens, M. J., Culp, S., Zaczek, R., Contrera, J. F., Bissette, G., Nemeroff, C. B., and De Souza, E. B., 1991. Role for brain corticotropin-releasing factor in the weight-reducing effects of chronic fenfluramine treatment in rats. Endocrinol. 128:3237-3246.
Richard, D., Rivest, S., and Rivier, C., 1992. The 5-hydroxy-tryptamine agonist fenfluramine increase Fos-like immuno reactivity in the brain. Brain Res. 594:131-137.
Le Feuvre, R. A., Rothwell, N. J., and Stock, M. J., 1987. Activation of brown fat thermogenesis in response to central injection of corticotropin releasing hormone in the rat. Neuropharmacol. 26:1217-1221.
Dunn, A. J., and Berridge, C. W., 1990. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress response? Brain Res. Rev. 15:71-100.
Rothwell, N. J., 1989. CRF is involved in the pyrogenic and thermogenic effects of interleukin-1β in the rat. Amer. J. Physiol. 256:E111-115.
Swiergiel, A. H., and Dunn, A. J., in press. CRF-deficient mice respond like wild type mice to hypophagic stimuli. Pharmacol. Biochem. Behav.
Dunn, A. J., and Berridge, C. W., 1987. Corticotropin-releasing factor administration elicits a stress-like activation of cerebral catecholaminergic systems. Pharmacol. Biochem. Behav. 27:685-691.
Lavicky, J., and Dunn, A. J., 1993. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J. Neurochem. 60:602-612.
Price, M. L., Curtis, A. L., Kirby, L. G., Valentino, R. J., and Lucki, I., 1998. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacol. 18:492-502.
Carr, D. J. J., and Blalock, J. E., 1991. Neuropeptide hormones and receptors common to the immune and neuroendocrine systems: bidirectional pathway of intersystem communication, Pages 573-588, in Ader, R., Felten, D. L., and Cohen, N. (eds.), Psychoneuroimmunology, Academic Press, San Diego.
Goetzl, E. J., Turck, C. W., and Speedharan, S. P., 1991. Production and recognition of neuropeptides by cells of the immune system, Pages 263-282, in Ader, R., Felten, D. L., and Cohen, N. (eds.), Psychoneuroimmunology, Academic Press, San Diego.
Stein, C., Hassan, A. H. S., Przewlocki, R., Gramsch, C., Peter, K., and Herz, A., 1990. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc. Natl. Acad. Sci. USA. 87:5935-5939.
Czlonkowski, A., Stein, C., and Herz, A., 1993. Peripheral mechanisms of opioid antinociception in inflammation: involvement of cytokines. Europ. J. Pharmacol. 242:229-235.
Fagarasan, M. O., Eskay, R., and Axelrod, J., 1989. Interleukin 1 potentiates the secretion of B-endorphin induced by secretagogues in a mouse pituitary cell line (AtT-20). Proc. Natl. Acad. Sci. USA 86:2070-2073.
Bessler, H., Sztein, M. B., and Serrate, S. A., 1990. β-endorphin modulation of IL-1 induced IL-2 production. Immunopharmacol. 19:5-14.
Heijnen, C. J., Kavelaars, A., and Ballieux, R. B., 1991. β-endorphin: cytokine and neuropeptide. Immunol. Rev. 119:41-63.
De Simoni, M. G., 1997. The role of the brain in IL-6 modulation. Neuroimmunomodulation 4:207.
Manfredi, B., Sacerdote, P., Gaspani, L., Poli, V., and Panerai, A. E., 1998. IL-6 knock-out mice show modified basal immune functions, but normal immune responses to stress. Brain Behav. Immun. 12:201-211.
Dunn, A. J., and Kramarcy, N. R., 1984. Neurochemical responses in stress: relationships between the hypothalamic-pituitary-adrenal and catecholamine systems, Pages 455-515, in Iversen, L. L., Iversen, S. D., and Snyder, S. H. (eds.), Handbook of Psychopharmacology, Plenum Press, New York.
Pechnick, R. N., 1993. Effects of opioids on the hypothalamo-pituitary-adrenal axis. Annu. Rev. Pharmacol. Toxicol. 33:353-382.
Ceccatelli, S., and Orazzo, C., 1993. Effect of different types of stressors on peptide messenger ribonucleic acids in hypothalamic paraventricular nucleus. Acta Endocrinol. 128:485-492.
Merchenthaler, I., 1992. Enkephalin-immunoreactive neurons in the parvicellular subdivisions of the paraventricular nucleus project to the external zone of the median eminence. J. Comp. Neurol. 326:112-120.
Wang, X. Q., Imaki, T., Shibasaki, T., Yamauchi, N., and Demura, H., 1996. Intracerebroventricular administration of β-endorphin increases the expression of c-fos and of corticotropin-releasing factor messenger ribonucleic acid in the paraventricular nucleus of the rat. Brain Res. 707:189-195.
Borsook, D., and Hyman, S. E., 1995. Proenkephalin gene regulation in the neuroendocrine hypothalamus: a model of gene regulation in CNS. Am. J. Physiol. 269:E393-E408.
Schafer, M., Mousa, S. A., and Stein, C., 1997. Corticotropin-releasing factor in antinociception and inflammation. Europ. J. Pharmacol. 323:1-10.
Harsing, L. G. J., Yang, H. Y., and Costa, E., 1982. Accumulation of hypothalamic endorphins after repeated injections of anorectics which release serotonin. J. Pharmacol. Exptl. Therap. 223:689-694.
Kmieciak-Kolada, K., and Kowalski, J., 1986. Involvement of the central serotonergic system in the changes of leu-enkephalin level in discrete rat brain areas. Neuropeptides 7:351-360.
Majeed, N. H., Lason, W., Pizewlocka, B., and Pizwlocki, R., 1986. Involvement of endogenous opioid peptides in fenfluramine anorexia. Pharmacol. Biochem. Behav. 25:967-972.
Bloom, F., Battenberg, E., Rossier, J., Ling, N., and Guillemin, R., 1978. Neurons containing B-endorphin in rat brain exist separately from those containing enkephalin: Immunocytochemical studies. Proc. Natl. Acad. Sci. USA 75:1591-1595.
Morley, J. E., 1980. The neuroendocrine control of appetite: the role of the endogenous opiates, cholecystokinin, TRH, gamma-aminobutyric-acid and the diazepam receptor. Life Sci. 27:355-368.
Foresta, C., Mioni, R., and Scandellari, C., 1986. Evidence for serotonergic system involvement in opioid control of luteinizing hormone secretion in man. Clin. Endocrinol. 25:573-578.
Caron, R. W., and Deis, R. P., 1996. Participation of opioid and serotonergic systems in prolactin secretion induced by hypothalamic action of estradiol. Neuroendocrinol. 64:124-130.
Wang, Q. P., and Nakai, Y., 1994. The dorsal raphe: an important nucleus in pain modulation. Brain Res. Bull. 34:575-585.
Sher, E., Cesare, P., Codignola, A., Clementi, F., Tarroni, P., Pollo, A., Magnelli, V., and Carbone, E., 1996. Activation of δ-opioid receptors inhibits neuronal-like calcium channels and distal steps of CA2+-dependent secretion in human small-cell lung carcinoma cells. J. Neurosci. 16:3672-3684.
Kondo, Y., Ogawa, N., Asanuma, M., Hirata, H., Tanaka, K., Kawada, Y., and Mori, A., 1993. Regional changes in neuropeptide levels after 5,7-dihydroxytryptamine-induced serotonin depletion in the rat brain. J. Neural Transm. 92:151-157.
Morris, B. J., Reimer, S., Hollt, V., and Herz, A., 1988. Regulation of striatal prodynorphin mRNA levels by the raphe-striatal pathway. Brain Res. 464:15-22.
Daunais, J. B., Hart, S. L., Hedgecock-Rowe, A., Matasi, J. J., Thornley, C., Davies, H. M., and Porrino, L. J., 1997. Alterations in behavior and opioid gene expression induced by the novel tropane analog WF-31. Molec. Brain Res. 50:293-304.
Akira, S., and Kishimoto, T., 1992. IL-6 and NF-IL6 in acutephase response and viral infection. Immunol. Rev. 127:25-50.
Klein, B., Zhang, X. G., Lu, Z. Y., and Bataille, R., 1995. Interleukin-6 in human multiple myeloma. Blood 85:863-872.
Vandenbeele, P., and Fiers, W., 1991. Is amyloidogenesis during Alzheimer's disease due to an IL-1/IL-6 mediated “acute phase response” in the brain? Immunol. Today 12:217-219.
Wood, J. A., Wood, P. L., Ryan, R., Graff-Radford, N. R., Pilapil, C., Robitaille, Y., and Quirion, R., 1993. Cytokine induces in Alzheimer's temporal cortex: no changes in mature IL-1β or IL-IRA but increases in the associated acute phase proteins IL-6, α2-macroglobulin and C-reactive protein. Brain Res. 629:245-252.
McClain, C., Cohen, D., Phillips, R., Ott, L., and Young, B., 1991. Increased plasma and ventricular fluid interleukin-6 levels in patients with head injury. J. Lab. Clin. Med. 118:225-231.
Hartung, H. P., Jung, S. F., Stoll, G., Zielasek, J., Schmidt, B., Archelos, J. J., and Toyka, K. V., 1992. Inflammatory mediators in demyelinating disorders of the CNS and PNS. J. Neuroimmunol. 40:197-210.
Fauci, A. S., 1993. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science 262:1011-1018.
Maes, M., 1995. Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuro-Psychopharmacol. Biol. Psychiat. 19:11-38.
Ganguli, R., Yang, Z. W., Shurin, G., Chengappa, K. N. R., Brar, J. S., Gubbi, A. V., and Rabin, B. S., 1994. Serum interleukin-6 concentration in schizophrenia-elevation associated with duration of illness. Psychiat. Res. 51:1-10.
Pomeroy, C., Eckert, E., Hu, S., Eiken, B., Mentink, M., Crosby, R. D., and Chao, C. C., 1994. Role of interleukin-6 and transforming growth factor-β in anorexia nervosa. Biol. Psychiat. 36:836-839.
Woodroofe, M. N., Sarna, G. S., Wadhwa, M., Hayes, G. M., Loughlin, A. J., Tinker, A., and Cuzner, M. L., 1991. Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J. Neuroimmunol. 33:227-236.
Rothwell, N. J., and Relton, J. K., 1993. Involvement of cytokines in acute neurodegeneration in the CNS. Neurosci. Biobehav. Rev. 17:217-227.
Jarskog, L. F., Xiao, H., Wilkie, M. B., Lauder, J. M., and Gilmore, J. H., 1997. Cytokine regulation of embryonic rat dopamine and serotonin neuronal survival in vitro. Intl. J. Dev. Neurosci. 15:711-716.
Lin, M. T., 1997. Heatstroke-reduced cerebral ischemia and neuronal damage. Involvement of cytokines and monoamines. Ann. N. Y. Acad. Sci. 813:572-580.
Campbell, L. L., Mucke, L., and Sandberg, K., 1993. Cerebral overexpression of interleukin-6 or interferon-α1 induces distinct neuropathology in transgenic mice. Soc. Neurosci. Abstr. 19:225.
Hart, B. L., 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12:123-137.
Kent, S., Bluthé, R.-M., Kelley, K. W., and Dantzer, R., 1992. Sickness behavior as a new target for drug development. TIPS 13:24-28.
Weingarten, H. P., 1996. Cytokines and food intake: the relevance of the immune system to the student of ingestive behavior. Neurosci. Biobehav. Rev. 20:163-170.
Swiergiel, A. H., Smagin, G. N., Johnson, L. J., and Dunn, A. J., 1997. The role of cytokines in the behavioral responses to endotoxin and influenza virus infection in mice: effects of acute and chronic administration of the interleukin-1-receptor antagonist (IL-1ra). Brain Res. 776:96-104.
Lenczowski, M. J. P., Bluthé, R. M., Roth, J., Rees, G. S., Rushforth, D. A., Van Dam, A. M., Tilders, F. J. H., Dantzer, R., Rothwell, N. J., and Luheshi, G. N., 1999. Central administration of rat interleukin-6 induces hypothalamus-pituitary-adrenal activation and fever but not sickness behavior in rats. Am. J. Physiol. in press
Plata-Salamán, C. R., 1998. Cytokine induced anorexia. Behavioral, cellular and molecular mechanisms. Ann. N.Y. Acad. Sci. 856
Swiergiel, A. H., Burunda, T., Peterson, B., and Dunn, A. J., 1999. Endotoxin-and interleukin-1-induced hypophagia are not affected by noradrenergic, dopaminergic, histaminergic and muscarinic antagonists. Pharmacol. Biochem. Behav. in press
Swiergiel, A. H., and Dunn, A. J., submitted for publication. Lack of evidence for a role of serotonin in interleukin-1-induced hypophagia. Pharmacol. Biochem. Behav.
Mantovani, G., Maccio, A., Lai, P., Massa, E., Ghiani, M., and Santona, M. C., 1998. Cytokine activity in cancer-related anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate. Semin. Oncol. 25:45-52.
Yamada, M., and Hatanaka, H., 1994. Interleukin-6 protects cultured rat hippocampal neurons against glutamate-induced cell death. Brain Res. 643:173-180.
Toulmond, S., Vige, X., Fage, D., and Benavidas, J., 1992. Local infusion of interleukin-6 attenuates the neurotoxic effects of NMDA on rat striatal cholinergic neurons. Neurosci. Letts 144:49-52.
Frei, K., Malipiero, U. V., Leist, T. P., Zinkernagel, R. M., Schwab, M. E., and Fontana, A., 1989. On the cellular source and function of interleukin-6 produced in the central nervous system in viral disease. Europ. J. Immunol. 19:689-694.
Maeda, Y., Matsumoto, M., Hori, O., Kuwabara, K., Ogawa, S., Yan, S. D., Ohtsuki, T., Kinoshita, T., Kamada, T., and Stern, D. M., 1994. Hypoxia/reoxygenation-mediated induction of astrocyte interleukin-6: a paracrine mechanism potentially enhancing neuron survival. J. Exptl. Med. 180:2297-2308.
Sei, Y., Vitkovic, L., and Yokoyama, M. M., 1995. Cytokines in the central nervous system: regulatory roles in neuronal function, cell death and repair. Neuroimmunomodulation 2:121-133.
Greenamyre, J. T., and Young, A. B., 1989. Excitatory amino acids and Alzheimer's disease. Neurobiol. Aging 10:593-602.
Choi, D. W., 1992. Excitotoxic cell death. J. Neurobiol. 23:1261-1276.
Lipton, S. A., 1992. Models of neuronal injury in AIDS: another role for the NMDA receptor? Trends Neurosci. 15:75-79.
Qui, Z., Parsons, K. L., and Gruol, D. L., 1995. Interleukin-6 selectively enhances the intracellular calcium response to NMDA in developing CNS neurons. J. Neurosci. 15:6688-6699.
Lipton, S. A., 1996. Similarity of neuronal cell injury and death in AIDS dementia and focal cerebral ischemia: potential treatment with NMDA open-channel blockers and nitric oxide-related species. Brain Pathol. 6:507-517.
Whitton, P. S., Richards, D. A., Biggs, C. S., and Fowler, L. J., 1994. N-Methyl-D-aspartate receptors modulate extracellular 5-hydroxytryptamine concentration in rat hippocampus and striatum in vivo. Neurosci. Letts. 169:215-218.
Aghajanian, G. K., and Marek, G. L., 1997. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacol. 36:589-599.
Robson, R. L., Westwick, J., and Brown, Z., 1995. Interleukin-1-induced IL-8 and IL-6 gene expression and production in human mesangial cells is differentially regulated by cAMP. Kidney Intl. 48:1767-1777.
Kiriyama, Y., Murayama, T., Tokumitsu, Y., and Nomura, Y., 1997. Protein kinase A-dependent IL-6 production induced by calcitonin in human glioblastoma A172 cells. J. Neuroimmunol. 76:139-144.
Itoi, K., Horiba, N., Tozawa, F., Sakai, Y., Sakai, K., Abe, K., Demur, H., and Suda, T., 1996. Major role of 3′,5′-cyclic adenosine monophosphate-dependent protein kinase A pathway in corticotropin-releasing factor gene expression in the rat hypothalamus in vivo. Endocrinol. 137:2389-2396.
Takao, T., Hashimoto, K., and De Souza, E. B., 1995. Modulation of interleukin-1 receptors in the brain-endocrine-immune axis by stress and infection. Brain Behav. Immun. 9:276-291.
Behan, D. P., Grigoriadis, D. E., Lovenberg, T., Chalmers, D., Heinrichs, S., Liaw, C., and DeSouza, E. B., 1996. Neurobiology of corticotropin releasing factor (CRF) receptors and CRF-binding protein: Implications for the treatment of CNS disorders. Molec. Psychiat. 1:265-277.
Heldwein, K. A., Redick, D. L., Rittenberg, M. B., Claycomb, W. C., and Stenzel-Poore, M. P., 1996. Corticotropin-releasing hormone receptor expression and functional coupling in neonatal cardiac myocytes and AT-1 cells. Endocrinol. 137:3631-3639.
Saitoh, M., Hasegawa, J., and Mashiba, H., 1990. Effect of corticotropin-releasing factor on the electrical and mechanical activities of the guinea-pig ventricular myocardium. Gen. Pharmacol. 21:337-342.
Vlaskovska, M., Schramm, M., Nylander, I., Kasakov, L., You, Z. B., Herrera-Marschitz, M., and Terenius, L., 1997. Opioid effects on 45CA2+ uptake and glutamate release in rat cerebral cortex in primary culture. J. Neurochem. 68:517-524.
Vaughan, C. W., and Christie, M. J., 1997. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J. Physiol. 498:463-472.
Mayer, D. L., Mao, J., and Price, D. D., 1995. The development of morphine tolerance and dependence is associated with translocation of protein kinase C. Pain 61:365-374.
Ventura, C., Maioli, M., Pintus, G., Posadino, A. M., and Tadolini, B., 1998. Nuclear opioid receptors activate opioid peptide gene transcription in isolated myocardial nuclei. J. Biol. Chem. 273:13383-13386.
Contesse, V., Hamel, C., Lefebvre, H., Dumuis, A., Vaudry, H., and Delarue, C., 1996. Activation of 5-hydroxytryptamine4 receptors causes calcium influx in adrenocortical cells: involvement of calcium in 5-hydroxytryptamine-induced steroid secretion. Molec. Pharmacol. 49:481-493.
Rorig, B., and Sutor, B., 1996. Regulation of gap junction coupling in the developing neocortex. Molec. Neurobiol. 12:225-249.
Chalecka-Franaszek, E., Chen, H., and Chuang, D.-M., 1999. 5-Hydroxytrytamine2A receptor stimulation induces activator protein-1 and cyclic AMP-responsive element binding with cyclic AMP-responsive element-binding protein and jun D as common components in cerebellar neurons. Neurosci. 88:885-898.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barkhudaryan, N., Dunn, A.J. Molecular Mechanisms of Actions of Interleukin-6 on the Brain, with Special Reference to Serotonin and the Hypothalamo-Pituitary-Adrenocortical Axis. Neurochem Res 24, 1169–1180 (1999). https://doi.org/10.1023/A:1020720722209
Issue Date:
DOI: https://doi.org/10.1023/A:1020720722209