Abstract
Purpose. Although the role of angiotensin-converting enzyme (ACE) inhibitors for the treatment of hypertension has been well established, no data has been generated regarding the influence of ACE inhibitors for health-related quality-of-life (QOL) dimensions for Chinese patients.
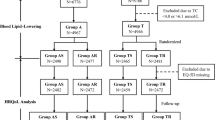
Materials. A double-blind, active-control, randomized clinical trial was used to compare the effects of two ACE inhibitors, imidapril and captopril, on quality-of-life dimensions in one outpatient clinic in one tertiary clinical-care facility. After a 2–3 week washout period with placebo, 59 patients with mild-to-moderate hypertension were randomly assigned to receive imidapril (5 to 10 mg per day) or captopril (25 to 50 mg twice per day) for 12 weeks. Patients completed the Short-form 36 (SF 36) health survey questionnaire, which evaluates 8 QOL dimensions, just before treatment, during the 8th week, and at the end of treatment (12th week). ANOVA for repeated measures was used to analyze the QOL-score changes over time and compare treatments, and to assess the interaction of treatment duration and group on these scores.
Results. No significant differences were demonstrated for changes in blood-pressure, frequency of adverse effects and withdrawal of patients from the study comparing the two drugs. Significant improvement, however, was demonstrated for mental-component summary scores after 12 weeks of treatment for both drugs (P = 0.029). No significant differences were established for individual QOL dimensions comparing the two drugs. A significantly higher baseline systolic blood pressure was found in the participants who did not complete the questionnaire than in those who did.
Conclusions. Similar and significant improvements were determined for the mental-component QOL summary scores for the two ACE inhibitors, imidapril and captopril, and no significant differences were demonstrated comparing treatments.
Similar content being viewed by others
References
Testa MA, Anderson RB, Nackley JF, Hollenberg NK. Quality of life and antihypertensive therapy in men. A comparison of captopril with enalapril. The Quality-of-Life Hypertension Study Group. New Eng J Med 1993;328:907–913.
Houston MC. The management of hypertension and associated risk factors for the prevention of long-term cardiac complications. J Cardiovasc Pharm 1993;21(Suppl 2):S2–S13.
Guidelines Subcommittee. 1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151–183.
Kubo M, Kato J, Ishida R, et al. Pharmacological studies on (4S)-1-methyl-3-(2S)-2-[N-((1S)-1-ethoxycarbonyl-3-phenylpropyl) amino] Propionyl-2-oxo-imidazolidine-4-carboxylic acid hydrochloride (TA-6366), a new inhibitor. I. ACE inhibitory and anti-hypertensive activities. Japan J Pharmacol 1990;53:201–210.
Omae T, Saruta T, Yoshinaga H, et al. Pilot study ofACE/TA-6366 (imidapril hydrochloride), an angiotensin-converting enzyme inhibitor, in patients with mild to moderate essential hypertension. J Clin Therap Med 1991;7:2187–2203.
Croog SH, Levine S, Testa MA, et al. The effects of antihypertensive therapy on the quality of life. N Eng J Med 1986;314:1657–1664.
Ferrara LA, Di Marino L, Russo O, Marotta T, Mancini M. Doxazosin and captopril in mildly hypercholesterolemic hypertensive patients. The Doxazosin-Captopril in Hypercholesterolemic Hypertensives Study. Hypertension 1993;21:97–104.
Neaton JD, Grimm RH Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study Research Group. Treatment of mild hypertension study: Final results.JAMA 1993;270:713–724.
Wassertheil-Smoller S, Blaufox D, Oberman A, et al. TAIM Research Group. Effect of antihypertensives on sexual function and quality of life: The TAIM study. Ann Intern Med 1991;114:613–620.
Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Medical Care 1989;27:217–233.
Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: Normative data for adults of working age. British Medical Journal 1993;306:1437–1440.
Jette DU, Downing J. Health status of individuals entering a cardiac rehabilitation program as measured by the medical outcomes study 36-item short-form survey (SF-36). Physical Therapy 1994;4:521–527.
Stewart AL, Hays RD, Ware JE. The MOS short-form general health survey reliability and validity in a patient population. Medical Care 1988;26:724–732.
Ware JE. Standards for validating health measures: Definition and content. J. Chron Dis 1987;40:473–482.
Wenger NK, Mattson ME, Furberg CD, et al. Assessment of quality of life in clinical trials of cardiovascular therapies. Am J Cardiol 1984;54:908–913.
Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Med Care 1992;30:473–483.
Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: The Health Institute, 1994.
Krousel-Wood MA, Re RN. Health status assessment in a hypertension section of an internal medicine clinic. Am J Med Sci 1994;308:211–217.
Huang PJ, Chien KL, ChenMF, Lai LP, Chiang FT. Efficacy and safety of imidapril in patients with essential hypertension: A double-blind comparison with captopril. Cardiology 2001;95:146–150.
Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide.Boston, MA: The Health Institute, 1993.
SAS Institute Inc. SAS/STAT User's Guide, Release 6.03 edition. 6.03 ed. Cary, NC: SAS Institute Inc. 1988.
Moser M. Angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists and calcium channel blocking agents: A review of potential benefits and possible adverse reactions. JACC 1997;29:1414–1421.
Meyer KB, Espindle DM, DeGiacomo JM, Jenuleson CS, Kurtin PS, Davis AR. Monitoring dialysis patients' health status. American Journal of Kidney Disease 1994;24:267–279.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chien, KL., Huang, PJ., Chen, MF. et al. Assessment of Quality of Life in a Double-Blind, Randomized Clinical Trial of Imidapril and Captopril for Hypertensive Chinese in Taiwan. Cardiovasc Drugs Ther 16, 221–226 (2002). https://doi.org/10.1023/A:1020648405680
Issue Date:
DOI: https://doi.org/10.1023/A:1020648405680