Abstract
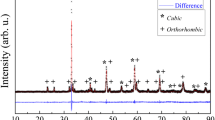
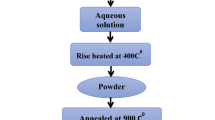
Li–Ni oxide mixtures with high lithium content are considered to be an alternative cathode material for molten carbonate fuel cells (MCFCs). The electrochemical behaviour of Li0.4Ni0.6O samples has been investigated in a Li–K carbonate melt at 650 °C by electrochemical impedance spectroscopy as a function of immersion time and O2 and CO2 partial pressure. The impedance spectra have been interpreted using a transmission line model that includes contact impedance between reactive particles. The Li0.4Ni0.6O powder particles show structural changes due to high lithium leakage and low nickel dissolution from the reactive surface to the electrolyte during the first 100 h of immersion. After this time, the structure seems to be stable. The partial pressures of O2 and CO2 affect the processes of oxygen reduction and Li–Ni oxide oxidation. X-ray diffraction and chemical analysis performed on samples before and after the electrochemical tests have confirmed that the lithium content decreases. SEM observations reveal a reduction in grain size after the electrochemical tests.
Similar content being viewed by others
References
T. Nishina, S. Ohuchi, K. Yamada and I. Uchida, J. Electroanal. Chem. 408 (1996) 181.
B. Fang and H. Chen, J. Electroanal. Chem. 501 (2001) 128.
M. Cassir and C. Belhomme, Plasma & Ions 1 (1999) 3.
M. Mohamedi, Y. Hisamitsu, Y. Ono, T. Itoh and I. Uchida, J. Appl. Electrochem. 30 (2000) 1397.
A. Prins-Jansen, G.A.J.M. Plevier, K. Hemmes and J.H.W. de Witt, Electrochim. Acta 41 (1996) 1323.
P. Tomczyk, J. Wyrna and M. Mosialek, J. Electroanal. Chem. 463 (1999) 78.
F.J. Pérez, D. Duday, M.P. Hierro, C. Gómez, M. Romero, M.T. Casais, J.A. Alonso, M.J. Martínez and L. Daza, J. Power Sources 72 (2000) 309.
T. Fukui, H. Okawa, T. Tsunooka, J. Power Sources 71 (1998) 239.
L. Plomb, E.F. Sitters, C. Vessies and F.C. Eckes, J. Electrochem. Soc. 138 (1991) 629.
R.C. Makkus, K. Hemmes and J.H.W. de Wit, J. Electrochem. Soc. 141 (1994) 429.
T. Fukui, S. Ohara, H. Okawa, T. Hotta and M. Naito, J. Power Sources 86 (2000) 340.
K. Hatoh, J. Nikura, E. Yasumoto and T. Gamo, Denki Kagaku 64 (1996) 825.
L. Daza, M.J. Escudero, E. Hontanñón, C.M. Rangel, T. Rodrigo, M.T. Casais and M.J. Martínez, 2000 Fuel Cell Seminar, Portland, Oregon, USA. Abstracts 30 Oct.–2 Nov. (2000) 732.
L. Giorgi, M. Carewska, S. Scaccia, E. Simoneti, E. Giacometti and R. Tulli, Int. J. Hydrogen Energy 21 (1996) 491.
J.A. Nelder and R. Mead. Computer J. 7 (1985) 308.
C.M. Abreu, M. Izquierdo, M. Keddam, X.R. Nóvoa and H. Takenouti, Electrochim. Acta 41 (1996) 2045.
M. Keddam, H. Takenouti, X.R. Nóvoa, C. Andrade and C. Alonso, Cem. Conc. Res. 27 (1997) 1191.
C. Belhomme, M. Cassir, J. Devynck and G. Gregoire, J. Mater. Sci. 35 (2000) 2683.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escudero, M., Nóvoa, X., Rodrigo, T. et al. Study of a Li–Ni oxide mixture as a novel cathode for molten carbonate fuel cells by electrochemical impedance spectroscopy. Journal of Applied Electrochemistry 32, 929–936 (2002). https://doi.org/10.1023/A:1020572308690
Issue Date:
DOI: https://doi.org/10.1023/A:1020572308690