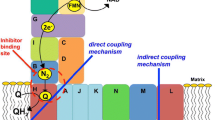
Abstract
Plasma membranes isolated from wild-type Saccharomyces cerevisiae crude membrane fractions catalyzed NADH oxidation using a variety of electron acceptors, such as ferricyanide, cytochrome c, and ascorbate free radical. Plasma membranes from the deletion mutant strain coq3Δ, defective in coenzyme Q (ubiquinone) biosynthesis, were completely devoid of coenzyme Q6 and contained greatly diminished levels of NADH–ascorbate free radical reductase activity (about 10% of wild-type yeasts). In contrast, the lack of coenzyme Q6 in these membranes resulted in only a partial inhibition of either the ferricyanide or cytochrome-c reductase. Coenzyme Q dependence of ferricyanide and cytochrome-c reductases was based mainly on superoxide generation by one-electron reduction of quinones to semiquinones. Ascorbate free radical reductase was unique because it was highly dependent on coenzyme Q and did not involve superoxide since it was not affected by superoxide dismutase (SOD). Both coenzyme Q6 and NADH–ascorbate free radical reductase were rescued in plasma membranes derived from a strain obtained by transformation of the coq3Δ strain with a single-copy plasmid bearing the wild type COQ3 gene and in plasma membranes isolated form the coq3Δ strain grown in the presence of coenzyme Q6. The enzyme activity was inhibited by the quinone antagonists chloroquine and dicumarol, and after membrane solubilization with the nondenaturing detergent Zwittergent 3–14. The various inhibitors used did not affect residual ascorbate free radical reductase of the coq3Δ strain. Ascorbate free radical reductase was not altered significantly in mutants atp2Δ and cor1Δ which are also respiration-deficient but not defective in ubiquinone biosynthesis, demonstrating that the lack of ascorbate free radical reductase in coq3Δ mutants is related solely to the inability to synthesize ubiquinone and not to the respiratory-defective phenotype. For the first time, our results provide genetic evidence for the participation of ubiquinone in NADH–ascorbate free radical reductase, as a source of electrons for transmembrane ascorbate stabilization.
Similar content being viewed by others
REFERENCES
Asard, H., Caubergs, R., Renders, D., and De Greef, J. A. (1987). Plant Sci. 53, 109-119.
Clarke, C. F., Williams, W., and Teruya, J. H. (1991). J. Biol. Chem. 266, 16636-16644.
Do, T. Q., Schultz, J. R., and Clarke, C. F. (1996). Proc. Natl. Acad. Sci. U. S. 93, 7534-7539.
Forsmark-Andrée P., Dallner, G., and Ernster, L. (1995). Free Rad. Biol. Med. 19, 749-757.
Gómez-Díaz, C., Rodríguez-Aguilera, J. C., Barroso, M. P., Villalba, J. M., Navarro, F., Crane, F. L., and Navas, P. (1997a). J. Bioenerg. Biomembr. 29, 253-259.
Gómez-Díaz, C., Villalba, J. M., Pérez-Vicente, R., Crane, F. L., and Navas, P. (1997b). Biochem. Biophys. Res. Commun. 234, 79-81.
Kagan, V. E., Nohl, H., and Quinn, P. J. (1996). In Handbook of Antioxidants (Cadenas, E., and Packer, L., eds.), Marcel Dekker, New York, pp. 157-201.
Kaplan, J., and O'Halloran, T. V. (1996). Science 271, 1510-1512.
Kim, C., Crane, F. L., Becker, G. W., and Morré, D. J. (1995). Protoplasma 184, 111-117.
Larm, J. A., Vaillant, F., Linnane, A. W., and Lawen, A. (1994). J. Biol. Chem. 296, 30097-30100.
Lesuisse, E., and Labbe, P. (1992). Plant Physiol. 100, 769-777.
Lesuisse, E., Casteras-Simon, M., and Labbe, P. (1996). J. Biol. Chem. 271, 13578-13583.
Littarru, G. P., Battino, M., and Folkers, K. (1996). In Handbook of Antioxidants (Cadenas, E., and Packer, L., eds.), Marcel Dekker, New York, pp. 203-239.
Marbois, B. N., Hsu, A., Pillai, R., Colicelli, J., and Clarke, C. F. (1994). Gene 138, 213-217.
Nakamura, M., and Hayashi, T. (1994). J. Biochem. 115, 1141-1147.
Navarro, F., Villalba, J. M., Crane, F. L., Mackellar, W. C., and Navas, P. (1995). Biochem. Biophys. Res. Commun. 212, 138-143.
Navas, P., Estévez, A., Burón, M. I., Villalba, J. M., and Crane, F. L. (1988). Biochem. Biophys. Res. Commun. 154, 1029-1033.
Navas, P., Villalba, J. M., and Córdoba, F. (1994). Biochim. Biophys. Acta 1197, 1-13.
Preusch, P. C., Siegel, D., Gibson, N. W., and Ross, D. (1991). Free Rad. Biol. Med. 11, 77-80.
Repetto, B., and Tzagoloff, A. (1989). Mol. Cell. Biol. 9, 2695-2705.
Rodríguez-Aguilera, J. C., and Navas, P. (1994). J. Bioenerg. Biomembr. 26, 379-384.
Santos-Ocaña, C., Navas, P., Crane, F. L., and Córdoba, F. (1995). J. Bioenerg. Biomembr. 27, 597-603.
Santos-Ocaña, C., Córdoba, F., Crane, F. L., Clarke, C. F., and Navas, P. (1998). J. Biol. Chem. 273, 8099-8105.
Schweinzer, E., and Goldenberg, H. (1993). Eur. J. Biochem. 218, 1057-1062.
Serrano, R. (1988). Methods Enzymol. 157, 533-544.
Serrano, R., Montesinos, C., Roldán, M., Garrido, G., Ferguson, C., Leonard, K., Monk, B. C., Perlin, D. S., and Weiler, E. W. (1991). Biochim. Biophys. Acta 1062, 157-164.
Shirabe, K., Yubisui, T., Nishino, T., and Takeshita, M. (1991). J. Biol. Chem. 266, 7531-7536.
Sikorski, R. S., and Hieter, P. (1989). Genetics 122, 19-27.
Stearman, R., Yuan, D. S., Yamaguchi-Iwai, Y., Klausner, R. D., and Dancis, A. (1996). Science 271, 1552-1557.
Steck, T. L., and Kant, J. A. (1974). Methods Enzymol. 31, 172-180.
Stoscheck, C. M. (1990). Methods Enzymol. 182, 50-68.
Storrie, B., and Madden, E. A. (1990). Methods Enzymol. 182, 203-225.
Sun, I. L., Sun, E. E., Crane, F. L., Morré, D. J., Lindgren, A., and Löw, H. (1992). Proc. Natl. Acad. Sci. U.S. 89, 11126-11130.
Tzagoloff, A., and Dieckmann, C. L. (1990). Microbiol. Rev. 54, 211-225.
Tzagoloff, A., Akai, A., and Needleman, R. B. (1975a). J. Biol. Chem. 250, 8228-8235.
Tzagoloff, A., Akai, A., and Needleman, R. B. (1975b). J. Bacteriol. 122, 826-831.
Tzagoloff, A., Wu, M., and Crivellone, M. (1986). J. Biol. Chem. 261, 17163-17169.
Umizawa, C., and Kishi, T. (1989). In The Yeasts (Rose, A. H., and Harrison, J. S., eds.), Vol 3, Academic Press, London, pp. 457-488.
Villalba, J. M., Canalejo, A., Rodríguez-Aguilera, J. C., Burón, M. I., Morré, D. J., and Navas, P. (1993a). J. Bioenerg. Biomembr. 25, 411-417.
Villalba, J. M., Canalejo, A., Burón, M. I., Córdoba, F., and Navas, P. (1993b). Biochem. Biophys. Res. Commun. 192, 707-713.
Villalba, J. M., Navarro, F., Córdoba, F., Serrano, A., Arroyo, A., Crane, F. L., and Navas, P. (1995). Proc. Natl. Acad. Sci. U.S. 92, 4887-4891.
Rights and permissions
About this article
Cite this article
Santos–Ocaña, C., Villalba, J.M., Córdoba, F. et al. Genetic Evidence for Coenzyme Q Requirement in Plasma Membrane Electron Transport. J Bioenerg Biomembr 30, 465–475 (1998). https://doi.org/10.1023/A:1020542230308
Issue Date:
DOI: https://doi.org/10.1023/A:1020542230308