Abstract
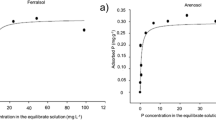
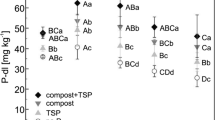
Phosphorus deficiency is one of the major constraints for normal plant growth and crop yields in the acid soils of Ghana and therefore addition of P inputs is required for sustainable crop production. This is often difficult, if not impossible for small-scale farmers due to the high cost of mineral P fertilizers and limited access to fertilizer supplies. Direct application of finely ground phosphate rocks (PRs) and their modified forms have been recommended as alternatives for P fertilization. The direct application of the natural and modified PRs to these acid soils implies the need to predict their agronomic effectiveness of the PRs in the simplest and most cost-effective manner. In this study the classical greenhouse pot experiment was compared to the 32P isotopic kinetics laboratory method for evaluating the agronomic effectiveness of natural and modified Togo PR in six highly weathered Oxisols from southwest Ghana. In the 32P isotopic kinetics laboratory experiment the six soil samples were each fertilised at the rate of 50 mg P kg−1 soil in the form of triple superphosphate (TSP), Togo PAPR-50%, and Togo PR, respectively. Controls without P amendment were also included. Isotopic exchange kinetics experiments were carried out on two sets of samples, immediately after P fertilizer additions (without incubation) and after 6 weeks of incubation under wet conditions and at a room temperature of 25 °C. In the greenhouse pot experiment, P fertilizers in the form of Togo PR, Togo PAPR, Mali PR and TSP were each applied to the six soils at rates equivalent to 0, 30, 60, and 120 kg P ha−1, respectively. The P fertilizers were mixed with the soils and maize (Zea mays L.) variety Obatanpa was grown for 42 days before harvest. The isotopic kinetics data of the control samples indicated that 5 of the studied soils had very low P fertility status as reflected by their low P concentrations in solution (CP<0.02 mg P l−1) and low exchangeable P (E1min < 5 mg P kg−1). The capacity factor and the fixation index of the soils were variable. Application of water-soluble P as TSP increased both the CP and E1 values of all the soils above the critical levels. Togo PR was least effective among the fertilizers tested for all soil soils, except in Boi soil. Acidulation of Togo PR (Togo PAPR-50%) was an effective means to increase its agronomic effectiveness. Direct application of natural Togo PR would be only feasible in the Boi soil series as reflected by its high Pdff% value in soil solution. Incubation with the P fertilizers caused an increase in the soil pH and a decline in the effectiveness of the applied P fertilizers, irrespective of the soil and the fertilizer utilized. Based upon the results of the greenhouse pot experiment, the relative crop response index (RCRI) in terms of increasing dry matter yield and P uptake followed the order of TSP > PAPR = Mali PR >Togo PR = Control. Both the laboratory index, Pdff% in soil solution derived from the isotopic method and the RCRI values obtained from the pot experiment produced similar results in ranking the P fertilizers tested according to their agronomic effectiveness. The isotopic kinetic method may be considered as an alternative to both greenhouse and field methods in the evaluation of agronomic effectiveness of P fertilizers in tropical acid soils when it offers comparative advantages in assessing the soil P status and its changes. But trained staff and adequate laboratory facilities are needed to perform this technique. Also the method can be used as a reference for comparison purposes as in this case. Further research is needed to assess the overall agronomic effectiveness (immediate and residual effects) of PR sources in predominant cropping systems of this region of Ghana.
Similar content being viewed by others
References
Abekoe MK & Tiessen H (1998) Fertilizer P transformations and P availability in hill slope soils of northern Ghana. Nutr Cycl Agroecosyst 52: 45–54
Allison LE, Bollen WB & Moodie CD (1965) Total Carbon. In: Black CA et al. (eds) Methods of Soil Analysis. Part 2, 1346–1365. Agronomy Monograph 9, Madison, WI
Barber SA (1984) Soil nutrient bioavailability: A mechanistic approach. New York: John Wiley and Sons
Bationo A, Chien SH, Henao J, Christianson CB & Mokwunye AU (1990) Agronomic evaluation of two unacidulated and partially acidulated phosphate rocks indigenous to Niger. Soil Sci Soc Am J 54: 1772–1777
Bear F (1964) Chemistry of the soil. American Chemical Society, Monograph series 160, 2nd edn. New York: Reinhold
Brammer H (1962) Soils of Ghana. In: Wills JB (ed) Agriculture and Land use in Ghana, pp 88–126. Oxford University Press, England
Bray RH & Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59: 39–45
Buresh RJ, Smithson PC & Hellums DT (1997) Building soil phosphorus capital in Africa. In: Buresh RJ, Sanchez PA & Calhoun F (eds) Replenishing soil fertility in Africa. SSSA Special Publication No. 51. Soil Science Society of America, Madison, Wisconsin, USA
Chien SH, Sale PWG & Friesen DK (1990) A discussion of the methods for comparing the relative effectiveness of phosphate fertilizers varying in solubility. Fert. Res. 24: 149–157
Chien SH & Hammond LL (1989) Agronomic effectiveness of partially acidulated phosphate rock as influenced by soil phosphorus-fixing capacity. Plant Soil 120: 159–164
Chien SH & Menon RG (1995) Factors affecting the agronomic effectiveness of phosphate rock for direct application. Fert. Res. 41: 227–234
Day PR (1965) Particle fractionation and particle-size analysis. In: Black CA et al. (eds) Methods of Soil Analysis. Part 1. 545–567 Agronomy Monogr. 9. ASA, Madison, WI
Fardeau JC & Frossard E (1992) Processus de transformation du phosphore dans les sols de l'Afrique de l'Ouest semi-aride: Application au phosphore assimilable. In: Tiessen H & Frossard E (eds) Phosphorus cycles in terrestrial and aquatic ecosystems. Regional workshop 4: Africa, pp 108–128. Saskatchewan Inst. of Pedology, Saskatoon
Fardeau JC, Guiraud G & Marol C (1995) Bioavailable soil P as a key to sustainable agriculture. Functional model determined by isotopic tracers. In: Proceedings of an International Symposium on Nuclear and related techniques in soil-plant studies on sustainable agriculture and environmental preservation. IAEA-SM-334/3. pp 131–144
Fardeau JC, Morel C & Boniface R (1991) Cinetiques de transfert des ions phosphates du sol vers la solution du sol. Agronomie 11: 577–584
Frossard E, Feller C, Tiessen H, Stewart JWB, Fardeau JC & Morel JL (1993) Can an isotopic method allow for the determination of the phosphate-fixing capacity of soils? Commun Soil Sci Plant Anal 24: 367–377
Hammond LL, Chien SH & Mokwunye AU (1986) Agronomic value of unacidulated and partially acidulated phosphate rocks indigenous to the tropics. Adv Agron 40: 89–140
Holford ICR (1997) Soil phosphorus: its measurement, and its uptake by plants. Aust J Soil Res 35: 227–239
Hue NV (1992) Correcting soil acidity of a highly weathered ultisol with chicken manure and sewage sludge. Commun Soil Sci Plant Anal 23: 241–264
IFDC (1999) African Fertilizer Market. Special Issue on Soil Fertility. Vol. 12,no. 12. IFDC Africa, Lome, Togo
Kanabo IAK & Gilkes RJ (1987a) A comparison between plant response and chemical measurements of the dissolution of reactive phosphate rock in soils of different pH and phosphorus retention. Aust J Soil Res 25: 451–460
Kanabo IAK & Gilkes R J (1987b) The role of soil pH in the dissolution of phosphate rock fertilizers. Fert Res 12: 165–179
Menon RG, Chien SH & Abd el Nabi Gadalla (1991) Phosphate rocks compacted with superphosphates vs partially acidulated rocks for bean and rice. Soil Sci Soc Am J 59: 1762–1767
Mokwunye AU (1995) Reactions in soils involving phosphate rocks. In: Gerner H & Mokwunye AU (eds) Use of Phosphate Rock for Sustainable Agriculture in West Africa, pp 84–91. IFDC-Africa
Morel C & Fardeau JC (1991) Phosphorus bioavailability of fertilizers: a predictive laboratory for its evaluation. Fert Res 28: 1–9
Murphy J & Riley J P (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27: 31–36
Owusu-Bennoah E, Szilas C, Hansen HCB & Borggaard OK (1997) Phosphate sorption in relation to aluminium and iron oxides of Oxisols from Ghana. Commun Soil Sci Plant Anal 28: 685–697
Rowell DL (1995) Soil Science: Methods and Applications. Longman Scientific and Technical, Harlow, Essex, England
Salcedo IH, Bertino F & Sampaio EVSB (1991) Assessing the reactivity of fertilizer P in tropical soils by isotopic exchange kinetics of 32P. Soil Sci Soc Am J 55: 140–145
Sinaj S, Frossard E & Fardeau JC (1997) Isotopically exchangeable phosphate in size fractionated and unfractionated soils. Soil Sci Am J 61: 1413–1417
Soil Survey Staff (1992) Keys to Soil Taxonomy. 5th edn. Soil Management Support Serv. Tech. 19. Pocahontas Press Inc., Blacksburgh, VA
Thomas GW (1982) Exchangeable cation. In: Page AL, Miller RH & Keeney SR (eds) Methods of Soil Analysis. Part 2, pp 159–166. ASA Monography 9. American Society of Agronomy, Madison, Wisconsin
Truong Binh, Pichot J & Beunard P (1978) Caracterisation et comparaison des phosphates naturels tricalciques d'Afrique de l'Ouest en vue de leur utilisation directe en agriculture. Agronomie Tropicale XXXIII-2: 136–145
Watanabe FS & Olsen SR (1965) Test of an ascorbic method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci Soc Am Proc 29: 677–678
Zapata F & Axmann H (1995) 32P isotopic techniques for evaluating the agronomic effectiveness of rock phosphate materials. Fert Res 41: 189–195
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Owusu-Bennoah, E., Zapata, F. & Fardeau, J. Comparison of greenhouse and 32P isotopic laboratory methods for evaluating the agronomic effectiveness of natural and modified rock phosphates in some acid soils of Ghana. Nutrient Cycling in Agroecosystems 63, 1–12 (2002). https://doi.org/10.1023/A:1020517221629
Issue Date:
DOI: https://doi.org/10.1023/A:1020517221629