Abstract
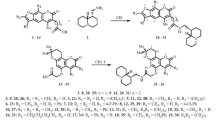
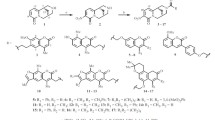
Psoralen and allopsoralen analogs that contain an annelated cyclopentane were synthesized from 7-hydroxyand 9-hydroxy-1,2,3,4-tetrahydrocyclopenta[c]chromen-4-ones. 9-Phenyl-1,2,3,4-tetrahydrocyclopenta[c]furo[3,2-g]chromen-4-one exhibited low toxicity, acted as a CNS stimulant, and exhibited cardiotropic activity.
Similar content being viewed by others
REFERENCES
Y. Kondo, A. Kato, Y. Kubota, and S. Nozoe, Heterocycles, 31, 187 (1990).
A. Hamed, I. Springuel, N. El-Amary, H. Mitome, and Y. Yamada, Phytochemistry, 1257 (1997).
M. D. Mashkovskii, Medicinal Preparations [in Russian], Gamta, Vilnius (1994), Vol. 2.
S. Kawaii, Y. Tomono, E. Katase, K. Ogawa, and M. Yano, Anticancer Res., 2505 (2000).
N. P. Maksyutina, N. F. Komisarenko, and A. F. Prokopenko, Plant Medicinal Preparations [in Russian], Zdorov′e, Kiev (1985).
A. Attia, A. M. Islam, A. M. El-Maghraby, and Y. Ammar, J. Prakt. Chem., 1039 (1979).
T. Okamoto, S. Yoshida, T. Kobayashi, and S. Okabe, Int. J. Mol. Med., 177 (2001).
O. H. Hishmat, A. H. Abd-El-Rahman, K. M. A. Khalil, M. I. Moawad, and M. M. Atalla, J. Pharm. Sci., 71, No. 9, 1046 (1982).
P. Zhou, Y. Takaishi, H. Duan, B. Chen, G. Honda, M. Itoh, Y. Takeda, O. K. Kodzhimatov, and K.-H. Lee, Phytochemistry, 53, 689 (2000).
Y. Shikishima, Y. Takaishi, G. Honda, M. Ito, Y. Takeda, O. K. Kodzhimatov, O. Ashurmetov, and K.-H. Lee, Chem. Pharm. Bull., 49, 877 (2001).
C. W. Holzapfel, P. S. Steyn, and I. F. H. Purchase, Tetrahedron Lett., 25, 2799 (1966).
U.S. Pat. No. 2860085; Chem. Abstr., 53, 9560d (1958).
Eur. Pat. No. 43535; Chem. Abstr., 97, 216033p (1982).
Ger. Offen. DE 3243158; Chem. Abstr., 101, 90767m (1984).
B. S. Verma, V. Abrol, N. K. Sanguan, and O. P. Malik, Chim. Acta Turc., 433 (1989).
Ger. Offen. DE 4111861; Chem. Abstr., 118, 124397j (1993).
Ger. Offen. DE 3834861; Chem. Abstr., 113, 171882v (1990).
E. C. Horning and D. E. Reisner, J. Am. Chem. Soc., 70, 3619 (1948).
J. K. MacLeod and M. Nakayama, Org. Mass Spectrom., 6, 293 (1972).
R. M. Naik and V. M. Thakor, J. Org. Chem., 22, 1696 (1957).
K. D. Kaufman, J. Org. Chem., 26, 117 (1961).
J. K. MacLeod, B. R. Worth, and R. J. Wells, Aust. J. Chem., 31, No. 7, 1533 (1978).
M. E. Perel′son, Yu. N. Sheinker, and A. A. Savina, Spectra and Structure of Coumarins, Chromones and Xanthones [in Russian], Meditsina, Moscow (1975).
D. Pillon, Bull. Soc. Chim. France, 324 (1952).
V. L. Dalla, O. Gia, G. Viola, G. Bertoloni, L. Santana, and E. Uriarte, Farmaco, 53, 638 (1998).
J. R. Merchant and S. M. Thakkar, Proc. Indian Acad. Sci., Sect. A, 82, 211 (1975).
Y. A. Shaikh and K. N. Trivedi, J. Indian Chem. Soc., 51, No. 8, 755 (1974).
P. B. Russell, A. R. Todd, S. Wilkinson, A. D. MacDonald, and G. Woolfe, J. Chem. Soc., 169 (1941).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garazd, M.M., Garazd, Y.L., Shilin, S.V. et al. Modified Coumarins. 4. Synthesis and Biological Properties of Cyclopentaneannelated Furocoumarins. Chemistry of Natural Compounds 38, 230–242 (2002). https://doi.org/10.1023/A:1020423826071
Issue Date:
DOI: https://doi.org/10.1023/A:1020423826071