Abstract
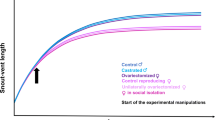
We address questions regarding the general absence of dimorphism in lemurid primates (Hapalemur, Eulemur, and Varecia) through comparative analyses of ontogeny. We described and analyzed body mass growth data for 9 lemurid taxa and compared them to similar data for anthropoid primates. Lemurids tend to grow rapidly over a short period of time when compared to anthropoid primates of similar body sizes. Size variation among lemurid taxa arises primarily as a consequence of differences in rates of growth. Comparative analyses of body mass growth data suggest that natural selection has produced ontogenetic adaptations in lemurids that center on relatively short periods of growth. Reduced growth periods preclude the evolution of sexual dimorphism through bimaturism—a sex difference in the length of the growth period—despite high levels of intermale competition. Selective factors related to seasonal variability of lemurid habitats play important roles in limiting the potential for the evolution of bimaturism. Other selective factors that limit bimaturism are related to female reproductive synchrony. In combination, they favor relatively early male maturation, precluding sexual selection that would otherwise promote the evolution of dimorphism through bimaturism. Natural selection on growth rates may preclude somatic responses to sexual selection that involve elevated male growth rates. In general, existing ontogenetic or life history adaptations appear to restrict responses to sexual selection in male lemurids.
Similar content being viewed by others
REFERENCES
Albrecht, G. H., Jenkins, P. D., and Godfrey, L. R. (1990). Ecogeographic size variation among the living and subfossil prosimians of Madagascar. Am. J. Primatol. 22: 1–50.
Arnold, S. J. (1983). Sexual selection, the interface of theory and empiricism. In Bateson, P. (ed.), Mate Choice, Cambridge University Press, Cambridge, pp. 181–210.
Bartlett, T. Q., Sussman, R. W., and Cheverud, J. M. (1993). Infant killing in primates: A review of observed cases with specific reference to the sexual selection hypothesis. Am. Anthropol. 95: 958–990.
Bell, G. (1980). The costs of reproduction and their consequences. Am. Nat. 116: 45–76.
Bicca-Marques, J. C., and Calegaro-Marques, C. (1998). Behavioral thermoregulation in a developmentally, sexually and dichromatic neotropical primate, the black-and-gold howling monkey (Alouatta caraya). Am. J. Phys. Anthropol. 106: 533–546.
Cheney, D. L., Seyfarth, R. M., Andelman, S. J., and Lee, P. C. (1988). Reproductive success in vervet monkeys. In Clutton-Brock, T. H. (ed.), Reproductive Success, University of Chicago, Chicago, pp. 384–402.
Cleveland, W. S. (1979). Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 74: 829–836.
Cleveland, W. S., and Devlin, S. J. (1988). Locally weighted regression: An approach to regression analysis by local fitting. J. Am. Stat. Assoc. 83: 596–610.
Clutton-Brock, T. H. (1985). Size, sexual dimorphism, and phylogeny in primates. In Jungers, W. L. (ed.), Size and Scaling in Primate Biology, Plenum Press, New York, pp. 51–60.
Clutton-Brock, T. H., Harvey, P. H., and Rudder, B. (1977). Sexual dimorphism, socionomic sex ratio, and body weight in primates. Nature 269: 191–195.
Colquhoun, I. D. (1987). Dominance and “fall fever”: The reproductive behavior of male brown lemurs (Lemur fulvus). Can. Rev. Phys. Anthropol. 6: 10–19.
Dunbar, R. I. M. (1987). Demography and reproduction. In Smuts, B. B., Cheney, D. L., Seyfarth, R. M., Wrangham, R. W., and Struhsaker, T. T. (eds.), Primate Societies, University of Chicago, Chicago, pp. 240–249.
Efron, B., and Tibshirani, R., (1991). Statistical data analysis in the computer age. Science 253: 390–395.
Felsenstein, J. (1985). Phylogenetics and the comparative method. Am. Nat. 126: 1–25.
Fisher, R. A. (1930). The Genetical Theory of Natural Selection, Oxford University Press, Oxford.
Garland, T., and Adolf, S. C. (1994). Why not to do two species comparative studies: Limitations on inferring adaptation. Physiol. Zool. 67: 797–828.
Godfrey, L. R., Sutherland, M. R., Petto, A. J., and Boy, D. S. (1990). Size, space, and adaptation in some subfossil lemurs from Madagascar. Am. J. Phys. Anthropol. 81: 45–66
Harvey, P. H., Martin, R. D., and Clutton-Brock, T. H. (1987). Life histories in comparative perspective. In Smuts, B. B., Cheney, D. L., Seyfarth, R. M., Wrangham, R. W., and Struhsaker, T. T. (eds.), Primate Societies, University of Chicago, Chicago, pp. 181–196.
Janson, C. H., and van Schaik, C. P. (1993). Ecological risk aversion in juvenile primates: Slow and steady wins the race. In Pereira, M. E., and Fairbanks, L. A. (eds.), Juvenile Primates, Oxford University Press, Oxford, pp. 57–76.
Jarman, P. J. (1983). Mating system and sexual dimorphism in large terrestrial mammalian herbivores. Biol. Rev. 58: 485–520.
Jolly, A. (1967). Breeding synchrony in wild Lemur catta. In Altmann, S. (ed.), Social Communication Among Primates, University of Chicago Press, Chicago, pp. 3–14.
Jolly, A. (1984). The puzzle of female feeding priority. In Small, M. (ed.), Female Primates: Studies by Women Primatologists, Alan R. Liss, New York, pp. 197–215.
Kappeler, P. M. (1990). The evolution of sexual size dimorphism in prosimian primates. Am. J. Primatol. 21: 201–214.
Kappeler, P. M. (1991). Patterns of sexual dimorphism in body weight among prosimian primates. Folia Primatol. 57: 132–146.
Kappeler, P. M. (1993). Sexual selection and lemur social systems. In Kappeler, P. M., and Ganzhorn, J.U. (eds.), Lemur Social Systems and Their Ecological Basis, Plenum Press, New York, pp. 223–240.
Kappeler, P. M., (1996). Causes and consequences of life-history variation among strepsirhine primates. Am. Nat. 148: 868–891.
Kappeler, P. M., and Ganzhorn, J. U. (1993). The evolution of primate communities and societies in Madagascar. Evol. Anthropol. 2: 151–188.
Kingsley, S. R. (1988). Physiological development of male orang-utans and gorillas. In Schwartz, J. H. (ed.), Orang-utan Biology, Oxford University Press, New York, pp. 123–132.
Lande, R. (1981). Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34: 292–305.
Leigh, S. R. (1992). Patterns of variation in the ontogeny of primate body size dimorphism. J. Hum. Evol. 23: 27–50.
Leigh, S. R. (1994a). The relations between captive and noncaptive weights in anthropoid primates. Zoo Biol. 13: 21–44.
Leigh, S. R. (1994b). The ontogenetic correlates of folivory in anthropoid primates. Am. J. Phys. Anthropol. 94: 499–522.
Leigh, S. R. (1995a). Socioecology and the ontogeny of sexual size dimorphism in anthropoid primates. Am. J. Phys. Anthropol. 97:339–356.
Leigh, S. R. (1995b). Ontogeny and the evolution of body size dimorphism in primates. Anthropologie (Brno) 33: 17–28.
Leigh, S.R. (1996). Evolution of human growth spurts. Am. J. Phys. Anthropol. 101: 455–474.
Leigh, S. R., and Shea, B. T. (1995). Ontogeny and the evolution of adult body size dimorphism in apes. Am. J. Primatol. 36: 37–60.
Martin, R. D. (1983) Human brain evolution in an ecological context. Fifty-second James Arthur Lecture on the Evolution of the Human Brain, 1982. American Museum of Natural History, New York.
Martin, R. D., Willner, L. A., and Dettling, A. (1994). The evolution of sexual size dimorphism in primates. In Short, R. V., and Balaban, E. (eds.), The Differences Between the Sexes, Cambridge University Press, Cambridge, pp. 159–202.
Neter, J., Wasserman, W., and Kutner, M. (1985). Applied Linear Statistical Models, Irwin Press, Homewood, IL.
Pereira, M. E. (1993). Seasonal adjustment of growth rate and adult body weight in ring-tailed lemurs. In Kappeler, P. M., and Ganzhorn, J. U. (eds.), Lemur Social Systems and Their Ecological Basis, Plenum Press, New York, pp. 205–222.
Plavcan, J. M., and van Schaik, C. P. (1997). Intrasexual competition and body weight dimorphism in anthropoid primates. Am. J. Phys. Anthropol. 103: 37–68.
Plavcan, J. M., van Schaik, C. P., and Kappeler, P. M., (1995). Competition, coalitions, and canine size in primates. J. Hum. Evol. 28: 245–276.
Promislow, D. E. L., and Harvey, P. H. (1990). Living fast and dying young: A comparative analysis of life history variation among mammals. J. Zool. 220: 417–437.
Ralls, K. (1977). Sexual dimorphism in mammals: Avian models and unanswered questions. Am. Nat. 111:917–938.
Ravosa, M.J., Meyers, D. M., and Glander, K. E. (1993). Relative growth of the limbs and trunk in sifakas: Heterochronic, ecological, and functional considerations. Am. J. Phys. Anthropol. 92:499–520.
Richard, A. F. (1974). Patterns of mating in Propithecus verreauxi. In Martin, R. D., Doyle, G. A., and Walker, A. C. (eds.), Prosimian Biology, Duckworth, London, pp. 49–75.
Richard, A. F. (1987). Malagasy prosimians: Female dominance. In Smuts, B. B., Cheney, D. L., Seyfarth, R. M., Wrangham, R. W., and Struhsaker, T. T. (eds.), Primate Societies, University of Chicago, Chicago, pp. 25–33.
Roberts, M. (1994). Growth, development, and parental care in the western tarsier (Tarsius banacanus) in captivity: Evidence for a “slow” life history and nonmonogamous mating system. Int. J. Primatol. 15: 1–28.
Rubenstein, D. I. (1993). On the evolution of juvenile life-styles in mammals. In Pereira, M. E., and Fairbanks, L. A. (eds.), Juvenile Primates, Oxford University Press, Oxford, pp. 38–56.
Sauther, M. L. (1993). Resource competition in wild populations of ring-tailed lemurs (Lemur catta): Implications for female dominance. In Kappeler, P. M., and Ganzhorn, J. U. (eds.), Lemur Social Systems and Their Ecological Basis, Plenum Press, New York, pp. 135–152.
Sauther, M. L., and Sussman, R. W. (1993). A new interpretation of the social organization and mating system of the ring-tailed lemur (Lemur catta). In Kappeler, P. M., and Ganzhorn, J. U. (eds.), Lemur Social Systems and Their Ecological Basis, Plenum Press, New York, pp. 111–122.
Seachrist, L. (1996). Chewing up the fossil record. Science 271: 1056.
Shea, B. T. (1986). Ontogenetic approaches to sexual dimorphism in anthropoids. Hum. Evol. 1: 97–110.
Stearns, S. C. (1992). The Evolution of Life Histories, Oxford University Press, Oxford.
Sussman, R. W. (1991). Demography and social organization of free-ranging Lemur catta in the Beza Mahafaly Reserve, Madagascar. Am. J. Phys. Anthropol. 84: 43–58.
Tattersall, I. (1982). The Primates of Madagascar, Columbia University Press, New York.
Terranova, C. J., and Coffman, B. S. (1997). Body weights of wild and captive lemurs. Zoo Biol. 16: 17–30.
van Schaik, C. P., and Kappeler, P. M. (1993). Life history, activity period, and lemur social systems. In Kappeler, P. M., and Ganzhorn, J. U. (eds.), Lemur Social Systems and Their Ecological Basis, Plenum Press, New York, pp. 241–260.
Wickings, E. J. (1995). Genetic self-management in a captive colony of mandrills (Mandrillus sphinx) as revealed by DNA minisatellite fingerprints. Electrophoresis 16: 167–168.
Wiley, R. H. (1974). Evolution of social organization and life history patterns among grouse. Q. Rev. Biol. 49: 201–226.
Wilkinson, L., Hill, M., Welna, J. P., and Birkenbeuel, G. K., (1992). Systat for Windows, Version 5, Systat, Evanston, IL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leigh, S.R., Terranova, C.J. Comparative Perspectives on Bimaturism, Ontogeny, and Dimorphism in Lemurid Primates. International Journal of Primatology 19, 723–749 (1998). https://doi.org/10.1023/A:1020381026848
Issue Date:
DOI: https://doi.org/10.1023/A:1020381026848