Abstract
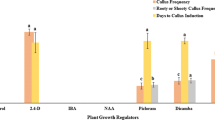
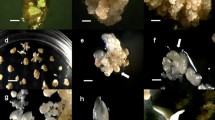
Optimization of the conditions for an efficient induction of somatic embryogenic calli and regeneration of plants from mature seeds of japonica rice cultivars was attempted. The number, color, size, shape, and appearance time of the induced embryogenic calli varied among the rice cultivars depending on the type of basal medium (LS, MS, N6). Presence of adequate amount of sucrose in the medium was an absolute requirement for embryogenic callus formation and shoot induction. Induction of the embryogenic calli, whose overall rates ranged from 30 to 56%, was most efficient in N6 medium supplemented with 3.0 mg l−1 of 2,4-D and 30 g l−1 of sucrose. Agar concentration in the regeneration medium was also critical for the shoot induction. Kinetin was found to be more effective for shoot regeneration compared with BA, while the highest shoot regeneration frequencies were observed when either cytokinin was combined with high concentration (2.0 mg l−1) of NAA. The optimal concentration of kinetin for the highest shoot regeneration frequency (67∼77%) was different among the cultivars tested. The embryogenic calli-derived shoots rooted on a plant growth regulator-free MS medium were successfully established in soil, producing fertile seeds.
Similar content being viewed by others
References
Abe T & Futsugara Y (1985) Efficient plant regeneration by somatic embryogenesis from root callus tissues of rice. J. Plant Physiol. 121: 111–118
Al-khayi JM, Shamblin CE, McNew RW & Anderson EJ (1996) Callus induction and plant regeneration of US rice genotype as affected by medium constituents. In Vitro Cell Dev. Biol. Plant. 32: 227–232
Christou P, Ford TL & Kofron M (1991) Production of transgenic rice (Oryza sativa L.) plants from agronomically important indica and japonica cultivars via electric discharge particle acceleration of exogenous DNA into immature zygotic embryo. Bio/technology 9: 957–962
Chu CC, Wang CC, Sun CS, Hus C, Yin KC & Chu CY (1975) Establishment of an efficient medium for another culture of rice through comparative experiments on the nitrogen sources. Sci. Sin. 18: 659–668
Constance N, Chowdhry, Tyagi AK, Maheshwary N & Mahseshwary SC (1993) Effect of L-proline and L-typtophan on somatic embryogenesis and plantlet regeneration of rice (Oryza sativa L. cv. Pusa 169). Plant Cell Tiss. Org. Cult. 32: 357–361
Datta SK, Datta K, Soltanifar N, Donn G & Potrykus I (1992) Herbicide resistant Indica rice plants from IRRI breeding line IR72 after PEG-mediated transformation of protoplast. Plant Mol. Biol. 20: 619–629
Garin E, Bernier-Cardou M, Isabel N, Klimaszewska K & Plourde A (2000) Effect of sugars, amino acids, and culture technique on maturation of somatic embryos of Pinus strobes on medium with two gellan gum concentrations. Plant Cell Tiss. Org. Cult. 62: 27–37
Heyser JW, Dykes TA, DeMott KJ & Nabors MW (1983) High frequency, long term regeneration of rice from callus culture. Plant Sci. Lett. 29: 175–182
Hiei Y, Ohta S, Komari T & Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium. and sequence analysis of the boundaries of the T-DNA. Plant J. 6: 271–282
Kamiya M, Yamanaka H & Oono Kiyoharu (1988) Intervarietal variations in somatic embryogenesis in rice (Oryza sativa L.). Bull. Natl. Inst. Agrobiol. Resour. 4: 127–151
Kishor PBK & Reddy GM (1986) Retention and revival of regenerating ability by osmotic adjustment in long-term cultures of four varieties of rice. J. Plant Physiol. 126: 49–54
Koetije DS, Grimes HD, Wang YC & Hodes TK (1989) Regeneration of indica rice (Oryza sativa L.) from primary callus derived from immature embryos. J. Plant Physiol. 135: 184–190
Komamine A, Kawahara R, Matsumoto M, Sunabori S, Toya T, Fujiwara A, Tsukahara M, Smith J, Ito M. Fukuda H, Nomura K & Fujimura T (1992) Mechanisms of somatic embryogenesis in cell cultures: physiology, biochemistry, and molecular biology. In Vitro Cell Dev. Biol. 28: 11–14
Li Z, Burow MD & Murai N (1990) High frequency generation of fertile transgenic rice plants after PEG-mediated protoplast transformation. Plant Mol. Biol. Rep. 8: 276–291
Linsmaier E & Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 18: 100–127
Masuda K, Kudo-Shiratori A & Inoue M (1989) Callus transformation and plant regeneration from rice protoplasts purified by density gradient centrifugation. Plant Sci. 62: 337–246
Morita M, Xing XH & Unno H (1999) Synchronized shoot regeneration of rice (Oryza sativa L.) calli on solid medium by adjustment of intracellular 2,4-dichlorophenoxyacetic acid concentration. Plant Cell Rep. 18: 633–639
Murashige T & Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Rueb S, Leneman M, Schilperoort RA & Hensgens LAM (1994) Efficient plant regeneration through somatic embryogenesis from callus induced on mature rice embryos (Oryza sativa L.). Plant Cell Tiss. Org. Cult. 36: 259–264
Tsugawa H & Suzuki M (2000) A low-temperature method for maintaining plant regeneration activity in embryogenic callus of rice (Oryza sativa L.). Plant Cell Rep. 19: 371–375
Yan CJ & Zhao QH (1982) Callus induction and plantlet regeneration from leaf blade of Oryza sativa L. subsp indica. Plant Sci. Lett. 29: 175–182
Ye X, Al-Babili S, Kloti A, Zhang J, Lucca, P, Beyer P & Potrykus I (2000) Engineering the provitamin A (ß -carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287: 303–305
Zhang W & Wu R (1988) Efficient regeneration of transgenic plants from rice protoplasts and correctly regulated expression of the foreign gene in the plants. Theor. Appl. Genet. 76: 835–840
Zhao J, Zhou C & Yang HY (1999) In vitro development of early proembryos and plant regeneration via microculture in Oryza sativa. Plant Cell Tiss. Org. Cult. 55: 167–174
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, K., Jeon, H. & Kim, M. Optimization of a mature embryo-based in vitro culture system for high-frequency somatic embryogenic callus induction and plant regeneration from japonica rice cultivars. Plant Cell, Tissue and Organ Culture 71, 237–244 (2002). https://doi.org/10.1023/A:1020305432315
Issue Date:
DOI: https://doi.org/10.1023/A:1020305432315