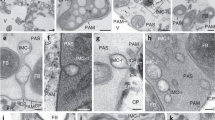
Abstract
Mycorrhizal fungi, to be effective for the plant, must be able to transfer mineral nutrient elements from sites of uptake at hyphal tips across various distances to the exchange region in the mycorrhiza. Vacuoles are likely to be important in this transport, since they contain elements of nutritional significance in abundance. In tip cells of hyphae of most fungi –- known to include three ectomycorrhizal basidiomycetes, an ericoid mycobiont, and two arbuscular mycorrhizal fungi –- the vacuoles form a motile tubular reticulum. The vacuoles are most active in hyphal tips, but non-motile vacuoles at a distance from the tip can be induced to become motile by environmental changes. Neither the tubular vacuolar reticulum nor its contents are properly preserved by conventional fixation and embedding. Vacuolar tubules are readily shown in vivo with fluorescent tracers, throughout the extramatrical mycelium and in outer hyphae of the sheath in eucalypt mycorrhizas synthesised with Pisolithus sp., but they have proved harder to label in field-collected ectomycorrhizas and ericoid mycorrhizas. Freeze-substitution does preserve the structure of vacuoles and vacuolar tubules, and careful anhydrous techniques allow them to be microanalysed, indicating high content of K and P in vacuoles of hyphal tips, and also in sheath and Hartig net of ectomycorrhizas. Vacuoles contain polyphosphate in diffuse, non-granular form. Polyphosphate is present right up to the tip region of hyphae as well as in sheath and Hartig net: thus important mineral nutrient elements are present at both ends of the long hyphal transport pathway. Exactly what happens in between, however, remains to be elucidated.
Similar content being viewed by others
References
Allaway W G and Ashford A E 2001 Motile tubular vacuoles in extramatrical mycelium and sheath hyphae of ectomycorrhizal systems. Protoplasma 215, 218-225.
Ashford A E 1998 Dynamic pleiomorphic vacuole systems: are they endosomes and transport compartments in fungal hyphae? Adv. Bot. Res. 28, 119-159.
Ashford A E and Orlovich D A 1994 Vacuole transport, phosphorus and endosomes in the growing tips of fungal hyphae. In Pollen-Pistil Interactions and Pollen Tube Growth, Current Topics in Plant Physiology 12. Eds. A G Stephenson and T-h Kao American Soc. Plant Physiologists, Maryland, USA.
Ashford A E Ling Lee M and Chilvers G A 1975 Polyphosphate in eucalypt mycorrhizas; a cytochemical demonstration. New Phytol. 74, 447-453.
Ashford A E, Peterson R L, Dwarte D and Chilvers G A 1986 Polyphosphate granules in eucalypt mycorrhizas: determination by energy dispersive x-ray microanalysis. Can. J. Bot. 64, 677-687.
Ashford A E, Ryde S and Barrow K D 1994 Demonstration of a short chain polyphosphate in Pisolithus tinctorius and the implications for phosphorus transport. New Phytol. 126, 239-247.
Ashford A E, Vesk P A, Orlovich D A, Markovina A-L and Allaway W G 1999 Dispersed polyphosphate in fungal vacuoles of Eucalyptus pilularis/Pisolithus tinctorius ectomycorrhizas. Fungal Genet. Biol. 28, 21-33.
Ashford A E, Cole L and Hyde G J 2001 Motile tubular vacuole systems. Ch 12. In The Mycota, VIII. Biology of the Fungal Cell Eds. R J Howard and N A R Gow. pp 243-265. Springer Ver, Heidelberg.
Bending G D and Read D J 1995a The structure and function of the vegetative mycelium of ectomycorrhizal plants V. Foraging behaviour and translocation of nutrients from exploited litter. New Phytol. 130, 401-409.
Bending G D and Read D J 1995b The structure and function of the vegetative mycelium of ectomycorrhizal plants VI. Activities of nutrient solubilizing enzymes in birch litter colonized by Paxillus involutus (Fr.) Fr. New Phytol. 130, 411-417.
Brandes B, Godbold D L, Kuhn A J and Jentschke G 1998 Nitrogen and phosphorus acquisition by the mycelium of the ectomycorrhizal fungus Paxillus involutus and its effect on host nutrition. New Phytol. 140, 735-743.
Bücking H and Heyser W 1999 Elemental composition and function of polyphosphates in ectomycorrhizal fungi-an x-ray microanalytical study. Mycol. Res. 103, 31-39.
Bücking H and Heyser W 2000a Subcellular compartmentation of elements in non-mycorrhizal and mycorrhizal roots of Pinus sylvestris: an X-ray microanalytical study. I. The distribution of phosphate. New Phytol. 145, 311-320.
Bücking H and Heyser W 2000b Subcellular compartmentation of elements in non-mycorrhizal and mycorrhizal roots of Pinus sylvestris: an X-ray microanalytical study. II. The distribution of calcium, potassium and sodium. New Phytol. 145, 321-331.
Cairney J W G and Smith S E 1992 Influence of intracellular phosphorus concentration on phosphate absorption by the ectomycorrhizal basidiomycete Pisolithus tinctorius. Mycol. Res. 96, 673-676.
Cole L, Hyde G J and Ashford A E 1997 Uptake and compartmentalisation of fluorescent probes by Pisolithus tinctorius hyphae: evidence for an anion transport mechanism at the tonoplast but not for fluid phase endocytosis. Protoplasma 199, 18-29.
Cole L, Orlovich D A and Ashford A E 1998 Structure, function, and motility of vacuoles in filamentous fungi. Fungal Genet. Biol. 24, 86-100.
Cole L, Davies D, Hyde G J and Ashford A E 2000a ER-tracker dye and BODIPY-brefeldin A differentiate the endoplasmic reticulum and Golgi bodies from the tubular-vacuole system in living hyphae of Pisolithus tinctorius. J. Microsc. 197, 239-248.
Cole L, Davies D, Hyde G J and Ashford A E 2000b Brefeldin A affects growth, endoplasmic reticulum, Golgi bodies, tubular vacuole system, and secretory pathway in Pisolithus tinctorius. Fungal Genet. Biol. 29, 95-106.
Frey B, Brunner I, Walther P, Scheidegger C and Zierold K 1997 Element localization in ultrathin cryosections of high-pressure frozen ectomycorrhizal spruce roots. Plant Cell Environ. 20, 929-937.
Haugland R P 2001 Handbook of Fluorescent Probes and Research Products, 8th ed. Molecular Probes Inc., Eugene, OR. http://www.probes.com
Hyde G and Ashford A E 1997 Vacuole motility and tubuleforming activity in Pisolithus tinctorius hyphae are modified by environmental conditions. Protoplasma 198: 85-92.
Hyde G, Davies D, Perasso L, Cole L and Ashford A E 1999 Microtubules but not actin filaments, regulate vacuole motility and morphology in hyphae of Pisolithus tinctorius. Cell Motil. Cytoskel. 42, 114-124.
Jentschke G, Brandes B, Kuhn AJ, Schröder W H, Godbold D L 2001 Interdependence of phosphorus, nitrogen, potassium and magnesium translocation by the ectomycorrhizal fungus Paxillus involutus. New Phytol. 149, 327-337.
Keenan K A, Utzat C D, Zielinski T K 1998 Isolation and characterization of strains defective in vacuolar ornithine permease in Neurospora crassa. Fungal Genet. Biol. 23, 237-247.
Klionsky D J, Herman P K and Emr S D 1990 The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev. 54, 266-292.
Martin F 1985 15N-NMR studies of nitrogen assimilation and amino acid biosynthesis in ectomycorrhizal fungus Cenococcum graniforme. FEBS Lett. 182, 350-354.
Martin F 1991 Nuclear magnetic resonance studies in ectomycorrhizal fungi. Methods Microbiol. 23, 121-148.
Martin F, Marchal J-P, Timinska A, Canet D 1985 The metabolism and physical state of polyphosphates in ectomycorrhizal fungi. A 31P nuclear magnetic resonance study. New Phytol. 101, 275-290.
Ogawa N, DeRisi J and Brown P O 2000 New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol. Biol. Cell 11, 4309-4321.
Orlovich D A and Ashford A E 1993 Polyphosphate granules are an artefact of specimen preparation in the ectomycorrhizal fungus Pisolithus tinctorius. Protoplasma 173: 91-105.
Orlovich D A and Ashford A E 1995 Maintenance of ion distribution in frozen dextran droplets during freeze-substitution, embedding and X-ray microanalysis in anhydrous conditions. J. Microsc. 180, 117-126.
Pålsgård E Lindh U and Roomans G M 1994 Comparative study of freeze-substitution techniques for X-ray microanalysis of biological tissue. Microsc. Res. Tech. 28, 254-258.
Perez-Moreno J and Read D J 2000 Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants. New Phytol. 145, 301-309.
Pfeffer P E, Bago B and Shachar-Hill Y 2001 Exploring mycorrhizal function with NMR spectroscopy. New Phytol. 150, 543-553.
Read D J 1984 The structure and function of the vegetative mycelium of mycorrhizal roots. In: The Ecology and Physiology of the Fungal Mycelium. Eds. D H Jennings and A D M Rayner. pp. 215-240. Cambridge University Press, Cambridge.
Rees B, Shepherd V A and Ashford A E 1994 Presence of a motile tubular vacuole system in different phyla of fungi. Mycol. Res. 98: 985-992.
Rost FWD, Shepherd V A and Ashford A E 1995 Estimation of vacuolar pH in actively growing hyphae of the fungus Pisolithus tinctorius. Mycol. Res. 99, 549-553.
Shepherd V A, Orlovich D A and Ashford A E 1993a A dynamic continuum of pleiomorphic tubules and vacuoles in growing hyphae of a fungus. J. Cell Sci. 104, 495-507.
Shepherd V A, Orlovich D A and Ashford A E 1993b Cell-to-cell transport via motile tubules in growing hyphae of a fungus. J. Cell Sci. 105, 1173-1178.
Steinberg T M, Newman A S, Swanson J A and Silverstein S C 1987 Macrophages possess probenecid-inhibitable organic anion transporters that remove fluorescent dyes from the cytoplasmic matrix. J. Cell Biol. 105, 2695-2702.
Tagu D and Martin F 1995 Expressed sequence tags of randomly selected cDNA clones from Eucalyptus globulus-Pisolithus tinctorius ectomycorrhiza. Mol. Plant Microbe Interact. 8, 781-783.
Tagu D and Martin F 1996 Molecular analysis of cell wall proteins expressed during the early steps of ectomycorrhiza development. New Phytol. 133, 73-85.
Tagu D, Nasse B and Martin F 1996 Cloning and characterization of hydrophobins-encoding cDNAs from the ectomycorrhizal Basidiomycete Pisolithus tinctorius. Gene 168, 93-97.
Uetake Y, Kajima T, Ezawa T and Saito M 2001 Tubular vacuoles observed in Gigaspora margarita hyphae using laser scanning confocal microscopy. Abstracts ICOM3, Adelaide.
Vesk P A, Ashford A E, Markovina A-L and Allaway W G 2000 Apoplasmic barriers and their significance in the exodermis and sheath of Eucalyptus pilularis-Pisolithus tinctorius ectomycorrhizas. New Phytol. 145: 333-346.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashford, A.E., Allaway, W.G. The role of the motile tubular vacuole system in mycorrhizal fungi. Plant and Soil 244, 177–187 (2002). https://doi.org/10.1023/A:1020271121683
Issue Date:
DOI: https://doi.org/10.1023/A:1020271121683