Abstract
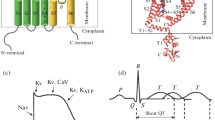
Potassium channels are a diverse class of transmembrane proteins that are responsible for diffusion of potassium ion across cell membranes. The lack of large quantities of these proteins from natural sources, is a major hindrance in their structural characterization using biophysical techniques. Synthetic peptide fragments corresponding to functionally important domains of these proteins provide an attractive approach towards characterizing the structural organization of these ion-channels. Conformational properties of peptides from three different potassium channels (Shaker, ROMK1 and minK) have been characterized in aqueous media, organic solvents and in phospholipid membranes. Techniques used for these studies include FTIR, CD and 2D-NMR spectroscopy. FTIR spectroscopy has been a particularly valuable tool for characterizing the folding of the ion-channel peptides in phospholipid membranes; the three different types of potassium channels all share a common transmembrane folding pattern that is composed of a predominantly α-helical structure. There is no evidence to suggest the presence of any significant β-sheet structure. These results are in excellent agreement with the crystal structure of a bacterial potassium channel (Doyle, D. A. et al. (1998) Science 280:69–77), and suggest that all potassium channel proteins may share a common folding motif where the ion-channel structure is constructed entirely from α-helices.
Similar content being viewed by others
REFERENCES
Aggeli, A. et al. (1998) Biochemistry 37:8121–8131.
Ben-Efraim, I. and Shai, Y. (1997) Biophys. J. 72:85–96.
Bogusz, S., Boxer, A., and Busath, D. D. (1992) Protein Engineering 5:285–293.
Brazier, S. P., Ramesh, B., Haris, P. I., Lee, D. C., and Srai, S. K. S. (1998) Biochem. J. 335:375–380.
Carpino, L. A. and Han, G. Y. (1972) J. Org. Chem. 37:3404–3409.
Cornell, B. A. et al. (1997) Nature 387:580–583.
Doyle, D. A. et al. (1998) Science 280:69–77.
Doak, D. G., Mulvey, D., Kawaguchi, K., Villalain, J., and Campbell, I. D. (1996) J. Mol. Biol. 258:672–687.
Durrell, S. R. and Guy, H. R. (1992) Biophysical Journal 62:238–250.
Durrell, S. R. and Guy, H. R. (1996) Neuropharmacol. 35:761–773.
Franciolini, F. (1994) Biochim. Biophys. Acta 1197:227–236.
Freeman, L. C. and Kass, R. S. (1993) Circ. Res. 73:968–973.
Ghadiri, M. R., Granja, J. R., Milligan, R. A., McRee, D. E., and Khazanovich, N. (1993) Nature 366:324–327.
Ghadiri, M. R., Granja, J. R., and Buehler, L. K. (1994) Nature 369:301–304.
Guy, H. R. and Durrell, S. R. (1994) Biophysical Journal 66:Abstr. 248.
Haris, P. I. and Chapman, D. (1992) Trends in Biochemical Science 17:328–333.
Haris, P. I., Lee, D. C., and Chapman, D. (1986) Biochim. Biophys. Acta 874:255–265.
Haris, P. I., Ramesh, B., Sansom, S. P., Kerr, I. D., Srai, K. S., and Chapman, D. (1994a) Protein Engineering 7:255–262.
Haris, P. I., Ramesh, B., Brazier, S., and Chapman, D. (1994b) FEBS Letters 349:371–374.
Haris, P. I., Ramesh, B., and Chapman, D. (1996) Prog. Biophys. and Mol. Biol. 65:PC312.
Haris, P. I. and Chapman, D. (1998a) in: Biomembrane Structures (Haris, P. I. and Chapman, D., eds.), IOS Press, Amsterdam, pp. 134–168.
Haris, P. I. and Chapman, D. (1998b) in: New Biomedical Materials (Haris, P. I. and Chapman, D., eds.), IOS Press, Amsterdam, pp. 24–31.
Haris, P. I., Wechselberger, R., and Czisch, M. (1998) Biochem. Soc. Trans. 26: S358. Structure of the S4 and S4–S5 loop region of a voltage-gated potassium channel.
Hartmann, H. A., Kirsch, G. E., Drewe, J. A., Taglialatela, M., Joho, R. H., and Brown, A. M. (1991) Science 251:942–944.
Heginbotham, L., Lu, Z., Abramson, T., and MacKinnon, R. (1994) Biophysical Journal 66:1061–1067.
Ho, K. et al. (1993) Nature 362:31–37.
Jackson, M., Haris, P. I., and Chapman, D. (1991) Biochemistry 30:9681–9686.
Kirsch, G. E. et al. (1992) Neuron 8:499–505.
Lopez, G. A., Jan, Y. N., and Jan, L. Y. (1994) Nature 367:179–182.
Lu, Q. and Miller, C. (1995) Science 268:310–317.
Mercer, E. A. J., Brazier, S., Abbott, G. W., Ramesh, B., Haris, P. I., and Srai, S. K. S. (1997) Biochem J. 325:475–479.
Merrifield, R. B. (1963) Journal of the American Chemical Society 85:2149–2154.
Montal, M. (1995) Ann. Rev. Biophys. Biomol. Struct. 24:31–57.
Pongs, O. (1992) Physiol. Rev. 72:S69–S88.
Pragnell, M. et al. (1990) Neuron 4:807–812.
Sanders, J. C., Haris, P. I., Chapman, D., Otto, C., and Hemminga, M. A. (1993) Biochemistry 32:12446–12454.
Sansom, M. S. P. (1991) Prog. Biophys. Molec. Biol. 55:139–235.
Slesinger, P., Jan, Y. N., and Jan, L. Y. (1993) Neuron 11:739–749.
Sugimoto, T. et al. (1990) J. Membr. Biol. 113:39–47.
Taglialatela, M., Wible, B. A., Caporaso, R., and Brown, A. M. (1994) Science 264:844–847.
Takumi, T., Ohkubo, H., and Nakanishi, S. (1988) Science 242:1042–1045.
Wilson, G. W., Sivaprasadarao, A., Findlay, J. B. C., and Wray, D. (1994) FEBS Lett. 353:251–254.
Yang, J., Jan, Y. N., and Jan, L. Y. (1995) Neuron 15:1441–1447.
Yellen, G., Jurman, M. E., Abramson, T., and MacKinnon, R. (1991) Science 251:939–942.
Yool, A. J. and Schwarz, T. L. (1991) Nature 349:700–704.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Haris, P.I. Synthetic Peptide Fragments as Probes for Structure Determination of Potassium Ion-Channel Proteins. Biosci Rep 18, 299–312 (1998). https://doi.org/10.1023/A:1020257215577
Issue Date:
DOI: https://doi.org/10.1023/A:1020257215577