Abstract
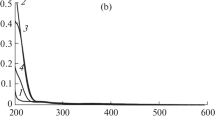
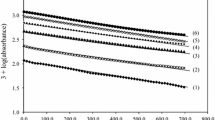
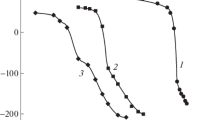
The kinetics of the electroreduction of palladium(II) complexes with α-alanine are studied on a dropping mercury electrode in solutions containing various supporting electrolytes (NaF, Na2SO4, NaClO4). The study is carried out at variable concentrations of palladium(II) alaninate complexes, the ligand, and the supporting electrolyte, in the pH interval extending from 3 to 11. The obtained values of the half-wave potential for the electroreduction of palladium(II) complexes with α-alanine and the diffusion coefficient for palladium(II) alaninate complexes suggest that, in the acidic and alkaline solutions studied, the slow electrochemical stage involves chelate dialaninate palladium complexes Pd(ala)2. Palladium(II) complexes underwent reduction on the positively-charged surface of DME without any preceding chemical stages. This process was hindered by anions of the supporting electrolyte adsorbed on DME, and in alkaline solutions, by the alaninate ions as well.
Similar content being viewed by others
REFERENCES
Kravtsov, V.I. and Shereshevskaya, I.I., Elektrokhimiya, 1971, vol. 7, p. 618.
Kravtsov, V.I. and Shereshevskaya, I.I., Elektrokhimiya, 1971, vol. 7, p. 99.
Kravtsov, V.I., Ivanov, V.D., Nikiforova, T.G., and Russkikh, Ya.V., Electrochim. Acta, 1997, vol. 42, p. 887.
Kravtsov, V.I. and Russkikh, Ya.V., Elektrokhimiya, 1997, vol. 33, p. 1007.
Kravtsov, V.I., J. Electroanal. Chem., 1976, vol. 69, p. 125.
Parry, E.P. and Oldham, K.B., Anal. Chem., 1968, vol. 40, p. 1031.
Tsventarnyi, E.G., Kravtsov, V.I., Russkikh, Ya.V., and Burogaa, K., Elektrokhimiya, 1997, vol. 33, p. 373.
Lai, C.K., Wang, Y.Y., and Wan, C.C., Bull. Chem. Soc. Jpn., 1991, vol. 64, p. 635.
Kurtova, O.Yu., Kravtsov, V.I., and Tsventarnyi, E.G., Elektrokhimiya, 2001, vol. 37, p. 1285.
Russkikh, Ya.V. and Kravtsov, V.I., Elektrokhimiya, 1997, vol. 33, p. 1240.
Mairanovskii, S.G., Elektrokhimiya, 1967, vol. 3, p. 1434.
Gergely, A., Magy. Kem. Foly., 1970, vol. 76, p. 452.
Griesser, R. and Sigel, H., Inorg. Chem., 1970, vol. 9, p. 1238.
Wada, G., Tamura, E., Okina, M., and Nakamura, M., Bull. Chem. Soc. Jpn., 1982, vol. 55, p. 3064.
Anderegg, J. and Malik, S., Helv. Chim. Acta, 1976, vol. 59, p. 1498.
Andrieux, C.P., Merz, A., and Saveant, J.-M., J. Am. Chem. Soc., 1985, vol. 107, p. 6097.
Saveant, J.-M., J. Am. Chem. Soc., 1987, vol. 109, p. 6788.
Payne, R., J. Phys. Chem., 1966, vol. 70, p. 204.
Parsons, R. and Payne, R., Z. Phys. Chem. (Munich), 1975, vol. 98, p. 98.
Ivanov, V.F., Damaskin, B.B., Segel'man, B.S., and Melekhova, N.I., Elektrokhimiya, 1973, vol. 9, p. 389.
Payne, R., J. Electroanal. Chem., 1975, vol. 60, p. 183.
Meites, L. and Israel, J., J. Am. Chem. Soc., 1961, vol. 83, p. 4903.
Koutecky, J., Collect. Czech. Chem. Commun., 1953, vol. 18, p. 597.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kravtsov, V.I., Nikiforova, T.G. Electroreduction Kinetics of Palladium(II) Complexes with α-Alanine: A Polarographic Study. Russian Journal of Electrochemistry 38, 972–980 (2002). https://doi.org/10.1023/A:1020236910932
Issue Date:
DOI: https://doi.org/10.1023/A:1020236910932