Abstract
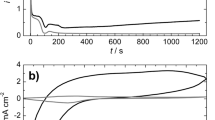
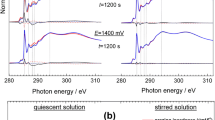
Copper deposition at polyaniline (PAN) coated electrodes is studied using copper oxalate complexes as reducing species. It is found that a high number (3.4 × 108 cm−2) of single sized (~150 nm), regular shaped crystals can be obtained. Statistical analysis of the distances between neighbouring crystals shows deviation from a random surface distribution. This finding is discussed both in terms of origin and overlap of nucleation exclusion zones and of the influence of the finite size of the copper crystals. Experiments performed under potentiostatic conditions give evidence for instantaneous copper nucleation and growth under phase boundary transition limitations. Results concerning number, size and shape of copper crystals deposited in a similar way using three different reducing species (i.e., copper cations, copper citrate and copper oxalate complex anions) are compared. It is established that the copper oxalate complex anions allow for deposition of the largest number of small metal crystals. This result is related to both the initial oxidation state of the PAN layer in the oxalate solution and to the specific properties of the anion complex.
Similar content being viewed by others
References
K.M. Kost, D.E. Bartak, B. Kazee and Th. Kuwana, Anal. Chem. 60 (1988) 2379.
S. Holdcroft and B. Lionel Funt, J. Electroanal. Chem. 240 (1988) 89.
P. Ocon Esteban, J.M. Leger and C. Lamy, J. Appl. Electrochem. 19 (1989) 462.
M. Gholamian and A.Q. Contractor, J. Electroanal. Chem. 289 (1990) 69.
Ch. Hable and M.S. Wrighton, Langmuir 9 (1993) 3284.
R. Kostecki, M. Ulmann, J. Augustynski, D.J. Strike and M. Koudelka-Hep, J. Phys. Chem. 97 (1993) 8113.
J-C. Moutet, Y. Ouennoughi, A. Ourari and S. Hamar-Thibault, Electrochim. Acta 40 (1995) 1827.
M. Hepel, J. Electrochem. Soc. 145 (1998) 124.
F. Ficicioglu and F. Kadirgan, J. Electroanal. Chem. 451 (1998) 95.
M.A. del Valle, F.R. Diaz, M.E. Bodini, T. Pizarro, R. Cordova, H. Gomez and R. Schrebler, J. Appl. Electrochem. 28 (1998) 943.
K. Bouzek, K-M. Mangold and K. Juettner, Electrochim. Acta 46 (2000) 661.
C. Coutanceau, M. J. Croissant, T. Napporn and C. Lamy, Electrochim. Acta 46 (2000) 579.
A. Yassar, J. Roncali and F. Garnier, J. Chem. Phys. 86 (1989) 241.
A. Zouaoui, O. Stephan, M. Carrier and J-C. Moutet, J. Electroanal. Chem. 474 (1999), 113.
S. Ivanov and V. Tsakova, J. Appl. Electrochem., this issue.
V. Tsakova, D. Borissov, B. Ranguelov, Ch. Stromberg and J.W. Schultze, Electrochim. Acta 46 (2001) 4213.
A.E. Martell and R.M. Smith, 'Critical Stability Constants', Vols 1–3, (Plenum Press, New York, 1977).
Gmelin's 'Handbuch der anorganischen Chemie', Vol. 60, Tl.B, Lfg.2, pp. 798–799.
A. Milchev, E. Vassileva and V. Kertov, J. Electroanal. Chem. 107 (1980) 323.
A. Milchev, Electrochim. Acta 28 (1983) 947.
A. Serruya, J. Mostany and B.R. Scharifker, J. Chem. Soc. Faraday Trans. 89 (1993) 255.
W.S. Kruijt, M. Sluyters-Rehbach, J.H. Sluyters and A. Milchev, J. Electroanal. Chem. 371 (1994) 13.
U. Scmidt, M. Donten and J.G. Osteryoung, J. Electrochem. Soc. 144 (1997) 2013.
W-S. Huang, B. Humphrey and A.G. Macdiarmid, J. Chem. Soc., Faraday Trans. 82 (1986) 2385.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ivanov, S., Tsakova, V. Influence of copper anion complexes on the incorporation of metal particles in polyaniline Part II: Copper oxalate complex. Journal of Applied Electrochemistry 32, 709–715 (2002). https://doi.org/10.1023/A:1020180703196
Issue Date:
DOI: https://doi.org/10.1023/A:1020180703196