Abstract
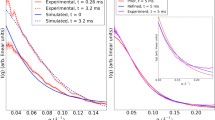
In this report, we addressed a somewhat basic question about how the twoextreme models, the all-atom model and the Ising-based model, can beconsistent with each other regarding the polypeptide helix-coil transition.Comparisons of several physical properties were made between the resultsof the all-atom simulations and those of the Ising-based theories. Fromthe equilibrium point of view, the two models were found to exhibit aqualitatively similar trend, which is significant considering the extremedifference in precision between the two models. On the other hand, fromthe kinetic viewpoint, there appeared a difference in relaxation behaviorbetween the two models; i.e., so-called stretched exponential relaxationwas observed in the all-atom simulation whereas the kinetic Ising modelshowed simple exponential relaxation. A plausible source of the observeddifference is briefly discussed.
Similar content being viewed by others
References
Poland, D. and Scheraga, H.A.: Theory of Helix-Coil Transitions in Biopolymers, Academic Press, New York, 1970, and the references therein.
Glauber, R.J.: Time-dependent statistics of the Ising model, J. Math. Phys. 4 (1963), 294–307.
Schwarz, G.: On the kinetics of the helix-coil transition of polypeptides in solution, J. Mol. Biol. 11 (1965), 64–77.
Takano, M., Takahashi, T. and Nagayama, K.: Helix-coil transition and 1 f fluctuation in a polypeptide, Phys.Rev.Lett. 80 (1998), 5691–5694.
Weiner, S.J., Kollman, P.A., Nguyen, D.T. and Case, D.A.: An all atom force field for simulations of proteins and nucleic acids, J. Comp. Chem. 7 (1986), 230–252.
Zimm, B.H. and Bragg, J.K.: Theory of the phase transition between helix and random coil in polypeptide chains, J. Chem. Phys. 31 (1959), 526–535.
Go, N.: The kinetics of the helix-coil transformation of polypeptides. I, J. Phys. Soc. Jpn. 22 (1967), 416–427.
Silberberg, A. and Simha, R.: On the kinetics of cooperative process. Closure approximations, Macromolecules 5 (1972), 332–334.
Tanaka, T., Wada, A. and Suzuki, M.: Dynamical aspects of helix-coil transitions in biopoly-mers. II, J. Chem. Phys. 59 (1973), 3799–3810.
Takano, M., Nagayama, K. and Suyama, A.: Investigating a link between all-atom model simu-lation and the Ising-based theory on the helix-coil transition: Equilibrium statistical mechanics. J. Chem. Phys. 116 (2002), 2219–2228.
Takano, M., Yamato, T., Higo, J., Suyama, A. and Nagayama, K.: Molecular dynamics of a 15-residue poly-L-alanine in water: Helix formation and energetics, J. Am. Chem. Soc. 121 (1999), 605–612.
Frauenfelder, H., Sligar, S.G. and Wolynes, P.G.: The energy landscapes and motions of proteins, Science 254 (1991), 1598–1603.
Schwarz Jr., M. and Poland, D.: Relaxation in biological macromolecules: Properties of some exact solutions, J. Chem. Phys. 65 (1976), 2620–2633.
Takano, M., Nagayama, K. and Suyama, A.: manuscript in preparation.
Rights and permissions
About this article
Cite this article
Takano, M., Nagayama, K. & Suyama, A. How the All-Atom Simulation and the Ising-Based Theory Reconcilewith Each Other on the Helix-Coil Transition. Journal of Biological Physics 28, 155–161 (2002). https://doi.org/10.1023/A:1019938505594
Issue Date:
DOI: https://doi.org/10.1023/A:1019938505594