Abstract
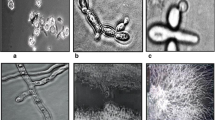
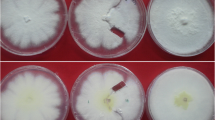
Mating types of Phaeosphaeria nodorum isolates (from North Africa, North America, Australia, Europe and Near East) were determined in laboratory conditions. Although both mating types were found, MAT1-1 and MAT1-2 were not evenly distributed among the isolates. Sexual compatibility pairings with standard mating type testers revealed that 56 of 101 isolates could be assigned to MAT1-1 and 5 to MAT1-2. Although the teleomorph occurred in different countries, the two mating types were observed only among isolates collected from France, Great Britain, Germany and Morocco. For Morocco, where no P. nodorum pseudothecia have been reported, this is the first report of the existence of the two opposite mating types. The remaining 40 isolates could not be designated to a specific mating type. However, in 17 crosses, pseudothecial initials or sterile pseudothecia were observed on wheat straw after two months. The implications of the predominance of one mating type are discussed.
Similar content being viewed by others
References
Allingham EA and Jackson LF (1981) Variation in pathogenicity, virulence, and aggressiveness of Septoria nodorum in Florida. Phytopathology 71: 1080–1085
Caten CE and Newton AC (2000) Variation in cultural characteristics, pathogenicity, vegetative compatibility and electrophoretic karyotype within field populations of Stagonospora nodorum. Plant Pathology 49: 219–226
Elmer WH (1995) A single population of Gibberella fujikuroi (Fusarium proliferatum) prodominates in asparagus fields in Connecticut, Massachusetts, and Michigan. Mycologia 87: 68–71
Fried PM and Meister E (1987) Inheritance of leaf and head resistance of winter wheat to Septoria nodorum in a diallel cross. Phytopathology 77: 1371–1375
Glass NL and Nelson MA (1994) Mating-Type genes in mycelial ascomycetes. In: Wessels JGH and Meinhardt F (ed) The Mycota: I. Growth, Differentiation and Sexuality. Springer-Verlag KG, Berlin
Halama P (1992) Observations sur l'hétérothallisme de Phaeosphaeria (Leptosphaeria) nodorum, agent de la septoriose du blé. Proceedings of the 44th International Symposium on Crop Protection, Medelingen van de Faculteit van de Landbouwwetenschappen, University of Gent, Belgium 57, 1304 pp
Halama P and Lacoste L (1991) Déterminisme de la reproduction sexuée de Phaeosphaeria (Leptosphaeria) nodorum, agent de la septoriose du blé. I Hétérothallisme et rôle des microspores. Canadian Journal of Botany 69: 95–99
Halama P and Lacoste L (1992a) Etude des conditions optimales permettant la pycniogénèse de Phaeosphaeria (Leptosphaeria) nodorum, agent de la septoriose du blé. Agronomie 12: 705–710
Halama P and Lacoste L (1992b) Déterminisme de la reproduction sexuée du Phaeosphaeria nodorum, agent de la septoriose du blé. II. Action de la temp´erature et de la lumière. Canadian Journal of Botany 70: 1563–1569
Halama P, Parguey Leduc A and Lacoste L (1992) Les organes de reproduction de l'Ascomycète Phaeosphaeria (Leptosphaeria) nodorum (E. Müller) Hedjaroude et de sa forme asexuée Septoria nodorum Berk. Canadian Journal of Botany 70: 1401–1408
Keller SM, McDermott JM., Pettway RE, Wolfe MS and McDonald BA (1997) Gene flow and sexual reproduction in the wheat glume blotch pathogen Phaeosphaeria nodorum (anamorph Stagonospora nodorum). Phytopathology 87: 353–358
Leslie JF and Raju NB (1985) Recessive mutations from natural populations of Neurospora crassa that are expressed in the sexual diplophase. Genetics 111: 759–777
McDonald BA, Miles J, Nelson LR and Pettway RE (1994) Genetic variability in nuclear DNA in field populations of Stagonospora nodorum. Phytopathology 84: 250–255
McGrath MT, Staniszewska H and Shishkoff N (1996) Distribution of mating types of Sphaerotheca fuliginea in the United States. Plant Disease 80: 1098–1102
Notteghem JL and Silué D (1992) Distribution of the mating type alleles in Magnaporthe grisea populations pathogenic on rice. Phytopathology 82: 421–424
Perkins DD (1994) How should the infertility of interspecific crosses be designated? Mycologia 86: 758–761
Rapilly F, Skajennikoff M, Halama P and Touraud G (1992) La reproduction sexuée et l'agressivité de Phaeosphaeria nodorum Hedj (Septoria nodorum Berk). Agronomie 12: 639–649
Scharen AL and Sanderson FR (1982) Leptosphaeria nodorum and Mycosphaerella graminicola in North America. Phytopathology 72: 934
Scharen AL, Eyal Z, Huffman MD and Prescott JM (1985) The distribution and frequency of virulence genes in geograhically separated populations of Leptosphaeria nodorum. Phytopathology 75: 1463–1468
Sharon A, Yamaguchi SV, Horwitz BA, Yoder OC and Turgeon BG (1996) An asexual fungus has the potential for sexual development. Molecular and General Genetics 251: 60–68
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Halama, P. Mating Relationships between Isolates of Phaeosphaeria nodorum, (anamorph Stagonospora nodorum) from Geographical Locations. European Journal of Plant Pathology 108, 593–596 (2002). https://doi.org/10.1023/A:1019933031308
Issue Date:
DOI: https://doi.org/10.1023/A:1019933031308