Abstract
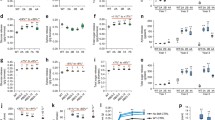
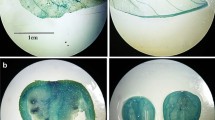
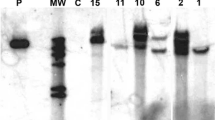
The function of the ripening-related endo-1,4-β-D-glucanase (EGase) CaCel1 in fruit softening was investigated by suppression of CaCel1 gene expression in transgenic pepper (Capsicum annuum L.) plants using constitutive expression of a truncated sense CaCel1 transgene. In suppressed lines, immunodetectable CaCel1 protein and extractable CMCase activity were reduced to at or below the limit of detection in ripe mature red fruit, suggesting that in pepper ripening-related CMCase activity is the product of a single gene. However, the abundances of two mRNAs derived from the CaCel1 gene by differential transcription initiation were affected differently in suppressed lines. Accumulation of a 1.7 kb CaCel1 transcript was strongly suppressed, whereas the abundance of a 2.1 kb CaCel1 transcript was only partially reduced. This implies that the 1.7 kb mRNA is responsible for producing CaCel1 protein, while the 2.1 kb mRNA is translationally inactive, and as such is recalcitrant to co-suppression. Chelator-soluble polyuronides exhibited little or no depolymerization during ripening, but matrix glycans including xyloglucan were extensively depolymerized. Depolymerization of non-xyloglucan matrix glycans was the prominant cell wall change observed during pepper ripening. However, the lack of CaCel1 activity in suppressed fruit had no detectable effect on ripening-related matrix glycan depolymerization, which occurred at wild-type levels. Recombinant CaCel1 protein purified from a transgenic pepper line over-expressing functional CaCel1 was active against pepper matrix glycansin vitro, and showed greater activity against non-xyloglucan polysaccharides than against xyloglucan. Transgenic suppression of CaCel1 EGase activity has not identified the natural cell wall substrate for this enzyme, and shows that activities other than CaCel1 are responsible for the depolymerization of matrix glycans occurring during ripening in pepper.
Similar content being viewed by others
References
Awad, M. and Young, R.E. 1979. Postharvest variation in cellulase, polygalacturonase, and pectinmethylesterase in avocado (Persea americana Mill, cv. Fuerte) fruits in relation to respiration and ethylene production. Plant Physiol. 64: 306–308.
Blumenkrantz, N. and Asboe-Hansen, G. 1973. New method for quantitative determination of uronic acids. Anal. Biochem. 54: 484–489.
Bonghi, C., Ferrarese, L., Ruperti, B., Tonutti, P. and Ramina, A. 1998. Endo-?-1,4-glucanases are involved in peach fruit growth and ripening, and regulated by ethylene. Physiol. Plant. 102: 346–352.
Brummell, D.A. and Harpster, M.H. 2001. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 47: 311–340.
Brummell, D.A., Lashbrook, C.C. and Bennett, A.B. 1994. Plant endo-1,4-?-D-glucanases: structure, properties, and physiological function. In: M.E. Himmel, J.O. Baker and R.P. Overend (Eds) Enzymatic Conversion of Biomass for Fuels Production (American Chemical Society Symposium Series 566), American Chemical Society, Washington DC, pp. 100–129.
Brummell, D.A., Hall, B.D. and Bennett, A.B. 1999a. Antisense suppression of tomato endo-1,4-?-glucanase Cel2 mRNA accumulation increases the force required to break fruit abscission zones but does not affect fruit softening. Plant Mol. Biol. 40: 615–622.
Brummell, D.A., Harpster, M.H., Civello, P.M., Palys, J.M., Bennett, A.B. and Dunsmuir, P. 1999b. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell 11: 2203–2216.
Carpita, N.C. and Gibeaut, D.M. 1993. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3: 1–30.
Cass, L.G., Kirven, K.A. and Christoffersen, R.E. 1990. Isolation and characterization of a cellulase gene family member expressed during avocado fruit ripening. Mol. Gen. Genet. 223: 76–86.
Dawson, D.M., Melton, L.D. and Watkins, C.B. 1992. Cell wall changes in nectarines (Prunus persica). Solubilization and depolymerization of pectic and neutral polymers during ripening and in mealy fruit. Plant Physiol. 100: 1203–1210.
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A. and Smith, F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28: 350–356.
Dunsmuir, P., Bond, D., Lee, K., Gidoni, D. and Townsend, J. 1987. The expression of introduced genes in regenerated plants. In: S.B. Gelvin and R.A. Schilperoort (Eds) Plant Molecular Biology Manual, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 45–59.
Engler, D.E., Guri, A.Z., Lauritis, J.A. and Schloemer, L.M.P. 1993. Genetically transformed pepper plants and methods for their production. U.S. Patent No. 5,262,316. Issued 16 November 1993.
Ferrarese, L., Trainotti, L., Moretto, P., Polverino de Laureto, P., Rascio, N. and Casadoro, G. 1995. Differential ethyleneinducible expression of cellulase in pepper plants. Plant Mol. Biol. 29: 735–747.
Ferrarese, L., Trainotti, L., Gattolin, S. and Casadoro, G. 1998. Secretion, purification and activity of two recombinant pepper endo-?-1,4-glucanases expressed in the yeast Pichia pastoris. FEBS Lett. 422: 23–26.
Firoozabady, E., Moy, Y., Tucker, W., Robinson, K. and Gutterson, N. 1995. Efficient transformation and regeneration of carnation cultivars using Agrobacterium. Mol. Breed. 1: 283–293.
Gross, K.C., Watada, A.E., Kang, M.S., Kim, S.D., Kim, K.S. and Lee, S.W. 1986. Biochemical changes associated with the ripening of hot pepper fruit. Physiol. Plant. 66: 31–36.
Harpster, M.H., Townsend, J.A., Jones, J.D.G., Bedbrook, J. and Dunsmuir, P. 1988. Relative strengths of the 35S cauliflower mosaic virus, 1', 2', and nopaline synthase promoters in transformed tobacco, sugarbeet, and oilseed rape callus tissue. Mol. Gen. Genet. 212: 182–190.
Harpster, M.H., Lee, K.Y. and Dunsmuir, P. 1997. Isolation and characterization of a gene encoding endo-?-1,4-glucanase from pepper (Capsicum annuum L.). Plant Mol. Biol. 33: 47–59.
Harpster, M.H., Brummell, D.A. and Dunsmuir, P. 1998. Expression analysis of a ripening-specific, auxin-repressed endo-1,4-?-glucanase gene in strawberry. Plant Physiol. 118: 1307–1316.
Hatfield, R. and Nevins, D.J. 1986. Characterization of the hydrolytic activity of avocado cellulase. Plant Cell Physiol. 27: 541–552.
Huber, D.J. 1984. Strawberry fruit softening: the potential roles of polyuronides and hemicelluloses. J. Food Sci. 49: 1310–1315.
Huber, D.J. and O'Donoghue, E.M. 1993. Polyuronides in avocado (Persea americana) and tomato (Lycopersicon esculentum) fruits exhibit markedly different patterns of molecular weight downshifts during ripening. Plant Physiol. 102: 473–480.
Kalaitzis, P., Hong, S.-B., Solomos, T. and Tucker, M.L. 1999. Molecular characterization of a tomato endo-?-1,4-glucanase gene expressed in mature pistils, abscission zones and fruit. Plant Cell Physiol. 40: 905–908.
Kanellis, A.K. and Kalaitzis, P. 1992. Cellulase occurs in multiple active forms in ripe avocado fruit mesocarp. Plant Physiol. 98: 530–534.
Kooiman, P. 1960. A method for the determination of amyloid in plant seeds. Recl. Trav. Chim. Pays-Bas 79: 675–678.
Lashbrook, C.C., Gonzalez-Bosch, C. and Bennett, A.B. 1994. Two divergent endo-?-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell 6: 1485–1493.
Lashbrook, C.C., Giovannoni, J.J., Hall, B.D., Fischer, R.L. and Bennett, A.B. 1998. Transgenic analysis of tomato endo-?-1,4-glucanase gene function. Role of cel1 in floral abscission. Plant J. 13: 303–310.
Levy, S., Maclachlan, G. and Staehelin, L.A. 1997. Xyloglucan sidechains modulate binding to cellulose during in vitro binding assays as predicted by conformational dynamics simulations. Plant J. 11: 373–386.
Lohmer, S., Maddaloni, M., Motto, M., Salamini, F. and Thompson, R.D. 1993. Translation of the mRNA of the maize transcriptional activator Opaque-2 is inhibited by upstream open reading frames present in the leader sequence. Plant Cell 5: 65–73.
Lumbreras, V., Campos, N. and Boronat, A. 1995. The use of an alternative promoter in the Arabidopsis thaliana HMG1 gene generates an mRNA that encodes a novel 3-hydroxy-3-355 methylglutaryl coenzyme A reductase isoform with an extended N-terminal region. Plant J. 8: 541–549.
Maclachlan, G. and Brady, C. 1994. Endo-1,4-?-glucanase, xyloglucanase and xyloglucan endo-transglycosylase activities versus potential substrates in ripening tomatoes. Plant Physiol. 105: 965–974.
McCollum, T.G., Huber, D.J. and Cantliffe, D.J. 1989. Modification of polyuronides and hemicelluloses during muskmelon fruit softening. Physiol. Plant. 76: 303–308.
O'Donoghue, E.M. and Huber, D.J. 1992. Modification of matrix polysaccharides during avocado (Persea americana) fruit ripening: an assessment of the role of Cx-cellulase. Physiol. Plant. 86: 33–42.
O'Donoghue, E.M., Huber, D.J., Timpa, J.D., Erdos, G.W. and Brecht, J.K. 1994. Influence of avocado (Persea americana) Cxcellulase on the structural features of avocado cellulose. Planta 194: 573–584.
Que, Q., Wang, H.-Y., English, J.J. and Jorgensen, R.A. 1997. The frequency and degree of cosuppression by sense chalcone synthase transgenes are dependent on transgene promoter strength and are reduced by premature nonsense codons in the transgene coding sequence. Plant Cell 9: 1357–1368.
Redgwell, R.J., Melton, L.D. and Brasch, D.J. 1991. Cell-wall polysaccharides of kiwifruit (Actinidia deliciosa): effect of ripening on the structural features of cell-wall materials. Carbohydr. Res. 209: 191–202.
Redgwell, R.J., Melton, L.D. and Brasch, D.J. 1992. Cell wall dissolution in ripening kiwifruit (Actinidia deliciosa). Solubilization of the pectic polymers. Plant Physiol. 98: 71–81.
Schibler, U. and Sierra, F. 1987. Alternative promoters in developmental gene expression. Annu. Rev. Genet. 21: 237–257.
Stam, M., de Bruin, R., Kenter, S., van der Hoorn, R.A.L., van Blokland, R., Mol, J.N.M. and Kooter, J.M. 1997. Post-transcriptional silencing of chalcone synthase in Petunia by inverted transgene repeats. Plant J. 12: 63–82.
Tong, C.B.S. and Gross, K.C. 1988. Glycosyl-linkage composition of tomato fruit cell wall hemicellulosic fractions during ripening. Physiol. Plant. 74: 365–370.
Wade, N.L., Kavanagh, E.E., Hockley, D.G. and Brady, C.J. 1992. Relationship beteen softening and the polyuronides in ripening banana fruit. J. Sci. Food Agric. 60: 61–68.
Wang, L. and Wessler, S.R. 1998. Inefficient reinitiation is responsible for upstream open reading frame-mediated translational repression of the maize R gene. Plant Cell 10: 1733–1745.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harpster, M.H., Brummell, D.A. & Dunsmuir, P. Suppression of a ripening-related endo-1,4-β-glucanase in transgenic pepper fruit does not prevent depolymerization of cell wall polysaccharides during ripening. Plant Mol Biol 50, 345–355 (2002). https://doi.org/10.1023/A:1019856929126
Issue Date:
DOI: https://doi.org/10.1023/A:1019856929126