Abstract
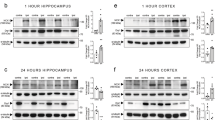
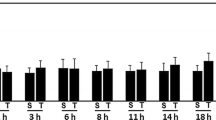
The inability to repair the damaged membrane may be one of the key mechanisms underlying the severe neuronal degeneration and overall functional loss seen in in vivo spinal cord injury and traumatic axonal injury in blunt head trauma. Promoting membrane resealing following damage may therefore constitute a potential effective therapeutic intervention in treating head trauma and spinal cord injuries. In our previous studies, we have shown that the axolemma failed to reseal following transection in clinically related situations, such as low extracellular calcium and low temperature. Our current studies indicate that DMSO is capable of rendering significant improvement in guinea pig axonal membrane resealing following transection in both 0.5 mM [Ca2+]0 and 25°C situations. This was demonstrated physiologically by monitoring membrane potential recovery and anatomically by conducting HRP-exclusion assays 60 minutes after injury. Further, we have shown that the addition of DMSO in normal Krebs' solution (2 mM [Ca2+]0 and 37°C) resulted in a decrease in membrane repair following injury. This indicates that DMSO-mediated membrane repair is sensitive to temperature and calcium. This study suggests the role of DMSO in axonal membrane resealing in clinically relevant conditions and raises the possibility of using DMSO in combination with other more established therapies in spinal cord injury treatment.
Similar content being viewed by others
References
Badylak, S. F., Babbs, C. F., Kougias, C. & Blaho, K. (1986) Effect of allopurinol and dimethylsulfoxide on long-term survival in rats after cardiorespiratory arrest and resuscitation. American Journal of Emergency Medicine 4, 313–318.
Brown, M., Desai, M., Traber, L. D., Herndon, D. N. & Traber, D. L. (1988) Dimethylsulfoxide with heparin in the treatment of smoke inhalation injury. Journal of Burn Care and Rehabilitation 9, 22–25.
Bruck, R., Aeed, H., Shirin, H., Matas, Z., Zaidel, L., Avni, Y. & Halpern, Z. (1999) The hydroxyl radical scavengers dimethylsulfoxide and dimethylthiourea protect rats against thioacetamide-induced fulminant hepatic failure. Journal of Hepatology 31, 27–38.
Buki, A., Siman, R., Trojanowski, J. Q. & Povlishock, J. T. (1999) The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. Journal of Neuropathology and Experimental Neurology 58, 365–375.
Clifton, G. L., Allen, S., Barrodale, P., Plenger, P., Berry, J., Koch, S., Fletcher, J., Hayes, R. L. & Choi, S. C. (1993) A phase II study of moderate hypothermia in severe brain injury. Journal of Neurotrauma 10, 263–271.
Cochran, T., Stefanko, J., Moore, C. & Saik, R. (1983) Dimethylsulfoxide protection against gastric stress ulceration. Current Surgery 40, 435–437.
Croall, D. E. & de Martino, G. N. (1991) Calciumactivated neurtal protease (calpain) system: Structure, function, and regulation. Physiological Reviews 71, 813–847.
Eddleman, C. S., Ballinger, M. L., Smyers, M. E., Fishman, H. M. & Bittner, G. D. (1998) Endocytotic formation of vesicles and other membranous structures induced by Ca2+ and axolemmal injury. Journal of Neuroscience 18, 4029–4041.
Fitzpatrick, M. O., Maxwell, W. L. & Graham, D. I. (1998) The role of the axolemma in the initiation of traumatically induced axonal injury [editorial]. Journal of Neurology, Neurosurgery and Psychiatry 64, 285–287.
Garrett, R. H. & Grisham, C. M. (eds) (1999) Biochemistry. Orlando: Saunders College Publishing.
Gitler, D. & Spira, M. E. (1998) Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron 20, 1123–1135.
Gross, G. W. & Higgins, M. L. (1987) Cytoplasmic damage gradients in dendrites after transection lesion. Experimental Brain Research 67, 52–61.
Hamakubo, T., Kannagi, R., Murachi, T. & Matus, A. (1986) Distribution of calpains I and II in rat brain. Journal of Neuroscience 6, 3103–3111.
Hansebout, R. R., Tanner, J. A. & Romerosierra, C. (1984) Current status of spinal cord cooling in the treatment of acute spinal cord injury. Spine 9, 508–511.
Hayes, K. C., Hsieh, J. T. C., Potter, P. J., Wolfe, D. L., Delaney, G. A. & Blight, A. B. (1993) Effects of induced hypothermia on somatosensory evoked potentials in patients with chronic spinal cord injury. Paraplegia 31, 730–741.
Heimer, L. & ZÁ borszky, L. (1989) Neuroanatomical Trac-Tracing Methods. New York: Plenum Press.
Howard, M. J., David, G. & Barrett, J. N. (1999) Resealing of transected myelinated mammalian axons in vivo: Evidence for involvement of calpain. Neuroscience 93, 807–815.
Kirpatovskii, V. I., Petrov D. A. & Kudriavtsev, Iu. V. (1995) The effect of an emulsion containing alphatocopherol and dimethylsulfoxide and of verapamil on reperfusion injury to the rat kidneys. Urologiia i Nefrologiia 32–35.
Koizumi, H. & Povlishock, J. T. (1998) Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. Journal of Neurosurgery 89, 303–309.
Lucas, J. H., Emery, D. G., Wang, G., Rosenbergschaffer, L. J., Jordan, R. S. & Gross, G. W. (1994) In vitro investigations of the effects of nonfreezing low temperatures on lesioned and uninjured mammalian spinal neurons. Journal of Neurotrauma 11, 35–61.
Lucas, J. H., Gross, G. W., Emery, D. G. & Gardner, C. R. (1985) Neuronal survival or death after dendrite transection close to the perikaryon: Correlation with electrophysiologic, morphologic, and ultrastructural changes. Central Nervous System Trauma 2, 231–255.
Luna, E. J & Hitt, A. L. (1992) Cytoskeleton-plasma membrane interactions. Science 258, 955–964.
Maxwell, W. L., Kosanlavit, R., McCreath, B. J., Reid, O. & Graham, D. I. (1999) Freeze-fracture and cytochemical evidence for structural and functional alteration in the axolemma and myelin sheath of adult guinea pig optic nerve fibers after stretch injury. Journal of Neurotrauma 16, 273–284.
Maxwell, W. L., McCreath, B. J., Graham, D. I. & Gennarelli, T. A. (1995) Cytochemical evidence for redistribution of membrane pump calcium-ATPase and ecto-Ca-ATPase activity, and calcium influx in myelinated nerve fibres of the optic nerve after stretch injury. Journal of Neurocytology 24, 925–942.
Maxwell, W. L., Povlishock, J. T. & Graham, D. L. (1997) A mechanistic analysis of nondisruptive axonal injury: A review [published erratum appears in J Neurotrauma 1997; 14(10), 755]. Journal of Neurotrauma 14, 419–440.
Maxwell, W. L., Watt, C., Graham, D. I. & Gennarelli, T. A. (1993) Ultrastructural evidence of axonal shearing as a result of lateral acceleration of the head in non-human primates. Acta Neuropathologica 86, 136–144.
McNeil, P. L. & Terasaki, M. (2001) Coping with the inevitable: How cells repair a torn surface membrane. Nature Cell Biology 3, 124–129.
Miyake, K., McNeil, P. L., Suzuki, K., Tsunoda, R. & Sugai, N. (2001) An actine barrier to resealing. Journal of Cell Science 114, 3487–3494.
Pettus, E. H., Christman, C. W., Giebel, M. L. & Povlishock, J. T. (1994) Traumatically induced altered membrane permeability: Its relationship to traumatically induced reactive axonal change. Journal of Neurotrauma 11, 507–522.
Pettus, E. H. & Povlishock, J. T. (1996) Characterization of a distinct set of intra-axonal ultrastructural changes associated with traumatically induced alteration in axolemmal permeability. Brain Research 722, 1–11.
Povlishock, J. T. (1992) Traumatically induced axonal injury: Pathogenesis and pathobiological implications. Brain Pathology 2, 1–12.
Povlishock, J. T. & Pettus, E. H. (1996) Traumatically induced axonal damage: Evidence for enduring changes in axolemmal permeability with associated cytoskeletal change. Acta Neurochirurgica-Supplementum 66, 81–86.
Robertson, C. L., Clark, R. S., Dixon, C. E., Alexander, H. L., Graham, S. H., Wisniewski, S. R., Marion, D. W., Safar, P. J. & Kochanek, P. M. (2000) No long-term benefit from hypothermia after severe traumatic brain injury with secondary insult in rats. Crititcal Care Medicine 28, 3218–3223.
Roederer, E., Goldberg, N. H. & Cohen, M. J. (1983) Modification of retrograde degeneration in transected spinal axons of the lamprey by applied DC current. Journal of Neuroscience 3, 153–160.
Saatman, K. E., Bozyczko-Coyne, D., Marcy, V., Siman, R. & McIntosh, T. K. (1996) Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. Journal of Neuropathology and Experimental Neurology 55, 850–860.
Sanger, J. W., Sanger, J. M., Kreis, T. E. & Jockusch, B. M. (1980) Reversible translocation of cytoplasmic actin into the nucleus caused by dimethyl sulfoxide. Proceedings of the National Academy of Sciences of the USA 77, 5268–5272.
Shi, R., Asano, T., Vining, N. C. & Blight, A. R. (2000) Control of membrane sealing in injured mammalian spinal cord axons. Journal of Neurophysiology 84, 1763–1769.
Shi, R. & Blight, A. R. (1996) Compression injury of mammalian spinal cord in vitro and the dynamics of action potential conduction failure. Journal of Neurophysiology 76, 1572–1580.
Shi, R. & Borgens, R. B. (2000) Anatomic repair of nervemembranein crushedmammalianspinal cordwith polyethylene glycol. Journal of Neurocytology 29, 633–644.
Shi, R. & Pryor, J. D. (2000) Temperature dependence of membrane sealing following transection in mammalian spinal cord axons. Neuroscince 98, 157–166.
Shiozaki, T., Kato, A., Taneda, M., Hayakata, T., Hashiguchi, N., Tanaka, H., Shimazu, T. & Sugimoto, H. (1999) Little benefit from mild hypothermia therapy for severely head injured patients with low intracranial pressure. Journal of Neurosurgery 91, 185–191.
Steinhardt, R. A., Bi, G. & Alderton, J. M. (1994) Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science 263, 390–393.
Stokes, B. T., Fox, P. & Hollinden, G. (1983) Extracellular calcium activity in the injured spinal cord. Experimental Neurology 80, 561–572.
Vincent, C., Pickering, S. J., Johnson, M. H. & Quick, S. J. (1990) Dimethylsulfoxide affects the organization of microfilaments in the mouse oocyte. Molecular Reproduction and Development 26, 227–235.
Westergren, H., Farooque, M., Olsson, Y. & Holtz, A. (2000) Motor function changes in the rat following severe spinal cord injury. Does treatment with moderate systemic hypothermia improve functional outcome? Acta Neurochirurgica 142, 567–573.
Winckler, B., Forscher, P. & Mellman, I. (1999) A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 397, 698–701.
Xie, X. & Barrett, J. N. (1991) Membrane resealing in cultured rat septal neurons after neurite transection: Evidence for enhancement by Ca2+-triggered protease activity and cytoskeletal disassembly. Journal of Neuroscience 11, 3257–3267.
Yawo, H. & Kuno, M. (1985) Calcium dependence of membrane sealing at the cut end of the cockroach giant axon. Journal of Neuroscience 5, 1626–1632.
Young, W., Yen, V. & Blight, A. (1982) Extracellular calcium ionic activity in experimental spinal cord contusion. Brain Research 253, 105–113.
Yu, Z. W. & Quinn, P. J. (1998) The modulation of membrane structure and stability by dimethyl sulphoxide (review). Molecular Membrane Biology 15, 59–68.
Rights and permissions
About this article
Cite this article
Shi, R., Qiao, X., Emerson, N. et al. Dimethylsulfoxide Enhances CNS Neuronal plasma membrane resealing after injury in low temperature or low calcium. J Neurocytol 30, 829–839 (2001). https://doi.org/10.1023/A:1019645505848
Issue Date:
DOI: https://doi.org/10.1023/A:1019645505848