Abstract
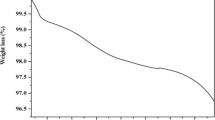
MnAPO‐11 samples were synthesized from aqueous (MnAPO‐11(A)) and ethylene glycol (MnAPO‐11(NA)) media. The crystallinity of the samples was more when the synthesis was carried out in ethylene glycol. Chemical and thermogravimetric analyses reveal greater incorporation of Mn in the framework of MnAPO‐11(NA) than in MnAPO‐11(A). At least five different types of Mn(II) species are detected in the samples by ESR. The studies suggest that Mn is more homogeneously distributed in MnAPO‐11(NA) than in MnAPO‐11(A).
Similar content being viewed by others
References
S.T. Wilson and E.M. Flanigen, US Patent 4567029 (1986).
C.A. Messina, B.M. Lok and E.M. Flanigen, US Patent 4544143 (1985).
C.J. Wright and N.B. Milestone, Eur. Patent Appl. 0141662 (1985).
D.R. Pyke, P. Whitney and H. Houghton, Appl. Catal. 18 (1985) 173.
G.C. Bond, M.R. Gelsthrope and K.S. Sing, J. Chem. Soc. Chem. Commun. (1985) 1056.
E.M. Flanigen, B.M. Lok, R.L. Patton and S.T. Wilson, Pure Appl. Chem. 58 (1986) 1351.
Z. Levi, A.M. Raitsimring and D. Goldfarb, J. Phys. Chem. 95 (1991) 7830.
C.W. Lee, X. Chen, G. Brouet and L. Kevan, J. Phys. Chem. 96 (1992) 3110.
G. Brouet, X. Chen, C.W. Lee and L. Kevan, J. Am. Chem. Soc. 114 (1992) 3720.
Z. Olender (Levi), D. Goldfarb and J. Batista, J. Am. Chem. Soc. 115 (1993) 1106.
N. Venkatathri, S.G. Hegde, P.R. Rajmohanan and S. Sivasanker, J. Chem. Soc. Faraday Trans. 93 (1997) 3411.
A.K. Sinha, S. Sivasanker and P. Ratnasamy, Ind. Eng. Chem. Res. 37 (1998) 2208.
A. Abragam and B. Bleary, in: Electron Paramagnetic Resonance of Transition Ions (Clarendon, Oxford, 1970) ch. 7, p. 378.
J.E. Wertz and J.E. Bolton, Electron Spin Resonance, Elementary Theory and Practical Applications (McGraw-Hill, New York, 1977) ch. 10, p. 223.
T.I. Barry and L.A. Lay, J. Phys. Chem. Solids 27 (1966) 1821.
T.I. Barry and L.A. Lay, Nature 208 (1965) 1312.
H.A. Kuska and M.T. Rogers, in: Radical Ions (Wiley/Interscience, New York, 1968) p. 579.
M. Goepper, F. Guth, L. Delmotte, J.L. Guth and H. Kessler, Stud. Surf. Sci. Catal. 49 (1989) 857.
J. Xu, Z. Luan, T. Wasowicz and L. Kevan, Micropor. Mesopor. Mater. 22 (1998) 179.
N.N. Tikhomirova and I.V. Nikolaeva, Russ. J. Phys. Chem. 55 (1981) 2224.
P.P. Knops-Gerrits, M. Labbe and P.A. Jacobs, Stud. Surf. Sci. Catal. 108 (1997) 445.
D.L. Griscom and R.E. Griscom, J. Chem. Phys. 47 (1967) 2711.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sinha, A., Jacob, N., Srinivas, D. et al. Location of Mn in MnAPO‐11: influence of synthesis from aqueous and non‐aqueous media. Catalysis Letters 61, 193–198 (1999). https://doi.org/10.1023/A:1019068704903
Issue Date:
DOI: https://doi.org/10.1023/A:1019068704903