Abstract
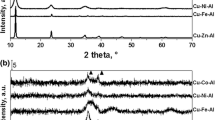
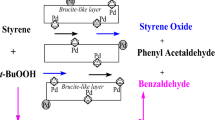
Various Mg-Al type hydrotalcites were examined as catalysts for the epoxidation of olefins and N-oxidation of pyridines using hydrogen peroxide. The catalytic activity of hydrotalcites increased with increasing the basicity of their surface. Adding cationic surfactants, e.g., n-dodecyltrimethylammonium bromide, to the above system remarkably accelerated the reaction rate. The hydrotalcites, into which were introduced both Ru and Co cations in the Brucite layers, were found to be good catalysts for the oxidation of various alcohols in the presence of molecular oxygen. Moreover, these hydrotalcites could smoothly catalyze also the oxygenation of diphenylmethane, fluorene, and xanthene at benzylic position with excellent yields. The hydrotalcite catalysts could be easily separated from the reaction mixture and reused with retention of their high catalytic performance for the above oxidations.
Similar content being viewed by others
References
T.J. Pinnavaia, Science 220 (1983) 365 (b)T. Nakamura and J.K. Thomas, Langmuir 3 (1987) 234 (c)K. Viaene, R.A. Schoonheydt, M. Crätzel, B. Kunyima and F.C. DeSchryver, Langmuir 4 (1988) 749 (d)J.K. Thomas, Acc. Chem. Res. 21 (1988) 275 (e)R.M. Barrer, Clays Clay Miner. 37 (1989) 385 (f)K. Takagi, T. Shichi, H. Usami and Y. Sawaki, J. Am. Chem. Soc. 115 (1993) 4339.
S. Miyata, T. Kumura, H. Hattori and K. Tanabe, Nippon Kagaku Zasshi 92 (1971) 514 (in Japanese) (b)S. Miyata, Clays Clay Miner. 23 (1975) 369 (c)S. Miyata, Clays Clay Miner. 28 (1980) 50 (d)S. Miyata, Clays Clay Miner. 31 (1983) 305 (e)W.T. Reichle, S.Y. Kang and D.S. Everhordt, J. Catal. 101 (1986) 352 (f)A. Drezdson, Inorg. Chem. 27 (1988) 4628 (g)F. Cavani, F. Trifirò and A. Voccari, Catal. Today 11 (1991) 173.
C.L. Hill, in: Advances in Oxygenated Processes, Vol. 1, eds. A.L. Baumstark (JAI Press, Inc., London, 1988) (b)M. Hudlucky, Oxidations in Organic Chemistry (American Chemical Society, Washington, DC, 1990).
S. Ueno, K. Yamaguchi, K. Yoshida, K. Ebitani and K. Kaneda, Chem. Commun. (1998) 295 (b)K. Yamaguchi, K. Ebitani and K. Kaneda, J. Org. Chem. 64 (1999) 2966.
K. Yamaguchi, T. Mizugaki, K. Ebitani and K. Kaneda, New J. Chem. 23 (1999) 799.
K. Kaneda, T. Yamashita, T. Matsushita and K. Ebitani, J. Org. Chem. 63 (1998) 1750 (b)T. Matsushita, K. Ebitani and K. Kaneda, Chem. Commun. (1999) 265.
B.M. Trost, Science 254 (1991) 1471 (b)P.T. Anastas and J.C. Warner, Green Chemistry: Theory and Practice (Oxford University Press, 1998) (c)J.H. Clark, Green Chem. 1 (1999) 1.
E. Weitz and A. Scheffer, Chem. Ber. 54 (1921) 2327 (b)E. Rohrmann, R.G. Jhones and H.A. Shonle, J. Am. Chem. Soc. 66 (1944) 1856.
G.B. Payne and P.H. Williams, J. Org. Chem. 24 (1959) 54 (b)G.B. Payne and P.H. Williams, J. Org. Chem. 26 (1961) 651 (c)G.B. Payne, Tetrahedron 19 (1962) 763.
T. Tatsumi, K. Yamamoto, H. Tajima and K. Tominaga, Chem. Lett. (1992) 815.
B.F. Sels, D.E.D. Vos and P.A. Jacobs, Tetrahedron Lett. 37 (1996) 8557.
C. Cativiela, F. Figueras, J.M. Fraile, J.I. Garcia and J.A. Mayoral, Tetrahedron Lett. 36 (1995) 4125 (b)J.M. Fraile, J.I. García, C. Cativiela, J.A. Mayoral and F. Figueras, Tetrahedron Lett. 37 (1996) 5995.
C.A. Bunton and G.J. Minkoff, J. Chem. Soc. (1949) 665.
B. Boyer, A. Hambardzoumian, G. Lamaty, A. Leydet, J.-P. Roque and P. Bouchet, New J. Chem. 20 (1996) 985.
L.J. Mathias and R.A. Vaidys, J. Am. Chem. Soc. 108 (1986) 1093 (b)S. Sakaguchi, Y. Nishiyama and Y. Ishii, J. Org. Chem. 61 (1996) 5307 (c)K. Sato, M. Aoki, M. Ogawa, T. Hashimoto, D. Panyella and R. Noyori, Bull. Chem. Soc. Jpn. 70 (1997) 905.
N. Lindquist, M.A. Battiste, W.M. Whitten, N.H. Williams and L. Strekowski, Phytochemistry 24 (1985) 683.
K. Kaneda, S. Ueno and T. Imanaka, J. Chem. Soc. Chem. Commun. (1994) 797 (b)K. Kaneda, S. Ueno and T. Imanaka, J. Mol. Catal. 102 (1995) 135 (c)S. Ueno, K. Ebitani, A. Ookubo and K. Kaneda, Appl. Surf. Sci. 121/122 (1997) 366.
I.E. Markó, P.R. Giles, M. Tsukazaki, S.M. Brown and C.J. Urch, Science 274 (1996) 2044 (b)I.E. Markó, P.R. Giles, M. Tsukazaki, I. Chellé-Regnaut, C.J. Urch and S.M. Brown, J. Am. Chem. Soc. 119 (1997) 12661 (c)A. Hanyu, E. Takezawa, S. Sakaguchi and Y. Ishii, Tetrahedron Lett. 39 (1998) 5557 (d)A. Dijksman, I.W.C.E. Arends and R.A. Sheldon, Chem. Commun. (1999) 1591 (e)T. Nishimura, T. Onoue, K. Ohe and S. Uemura, J. Org. Chem. 64 (1999) 6750 (f)G.-J. ten Brink, I.W.C.E. Arends and R.A. Sheldon, Science 287 (2000) 1636.
K. Kaneda, Y. Fujie and K. Ebitani, Tetrahedron Lett. 38 (1997) 9023 (b)K. Ebitani, Y. Fujie and K. Kaneda, Langmuir 15 (1999) 3557 (c)K. Yamaguchi, K. Mori, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc. 122 (2000) 7144 (d)T. Nishimura, N. Kakiuchi, M. Inoue and S. Uemura, J. Chem. Soc. Chem. Commun. (2000) 1245.
W.-H. Fung, W.-Y. Yu and C.-M. Che, J. Org. Chem. 63 (1998) 2873.
H.Y.H. Chan, C.G. Takoudis and M.J. Weaver, J. Catal. 172 (1997) 336.
M. Matsumoto and N. Watanabe, J. Org. Chem. 49 (1984) 3436.
A. Hanyu, E. Takezawa, S. Sakaguchi and Y. Ishii, Tetrahedron Lett. 39 (1998) 5557.
A. Dijksman, I.W.C.E. Arends and R.A. Sheldon, Chem. Commun. (1999) 1591.
K. Yamaguchi, K. Ebitani, T. Yoshida, H. Yoshida and K. Kaneda, J. Am. Chem. Soc. 121 (1999) 4526.
Rights and permissions
About this article
Cite this article
Kaneda, K., Yamaguchi, K., Mori, K. et al. Catalyst design of hydrotalcite compounds for efficient oxidations. Catalysis Surveys from Asia 4, 31–38 (2000). https://doi.org/10.1023/A:1019048407764
Issue Date:
DOI: https://doi.org/10.1023/A:1019048407764