Abstract
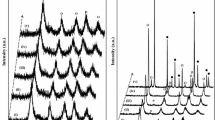
A series of polycrystalline WOx/TiO2 samples were prepared by means of a conventional impregnation method. The samples were characterized by means of X-ray diffraction, Vis-UV diffuse reflectance and Raman spectroscopies, nitrogen adsorption at 77 K for determining specific surface areas and surface texture, scanning electron microscopy, and FT-IR monitoring of pyridine adsorption for measuring the surface acidity. Catalytic activity of the samples has been assessed by carrying out as a ``probe'' reaction the photodegradation of 4- nitrophenol in aqueous medium. The results obtained indicate that incorporation of tungsten on titania leads to formation of different surface species, depending on the tungsten loading. Tungsta microcrystals were detected by X-ray diffraction when the nominal molar W/Ti ratio reached a value of 8.0%. FT-IR investigation indicated that the presence of tungsten induces formation of Brønsted and Lewis surface acid sites. The photoactivity results confirm the beneficial effect of tungsten in TiO2 for 4-nitrophenol photodegradation in aqueous medium. The reaction rates are higher than those reported in literature for another set of samples and maximum photoactivity was achieved for a sample containing 1.96 moles of W per 100 moles of Ti.
Similar content being viewed by others
References
M. Ai, J. Catal. 49 (1977) 305.
T. Yamaguchi, Y. Tanaka and K. Tanabe, J. Catal. 65 (1980) 442.
T. Yamaguchi, S. Nakamura and H. Naguno, in: Proc. 8th Int. Congr. on Catalysis, Vol. 5 (Verlag Chemie, Weinheim, 1984) p. 579.
M. Imanari, Y. Watanabe, S. Matsuda and F. Nakajima, in: Proc. 7th Int. Congr. on Catalysis, eds. T. Seiyama and K. Tanabe (Elsevier, Amsterdam, 1981) p. 841.
S. Morikawa, K. Takahashi, J. Mogi and S. Kurita, Bull. Chem. Soc. Jpn. 55 (1982) 2254.
W. Lee, W.M. Gao, K. Dwight and A. Wold, Mater. Res. Bull. 27 (1992) 685.
Y.R. Do, W. Lee, K. Dwight and A. Wold, J. Solid State Chem. 108 (1994) 198.
G. Marcì, L. Palmisano, A. Sclafani, A.M. Venezia, R. Campostrini, G. Carturan, C. Martìn and G. Solana, J. Chem. Soc. Faraday Trans. 92 (1996) 819.
G.A. Somorjai, Introduction to Surface Chemistry and Catalysis (Wiley, New York, 1994).
M. Schiavello, Electrochim. Acta 38 (1993) 11.
H. Bosch and E. Peppelenbos, J. Phys. E 10 (1977) 605.
JCPDS files 21-1276, 21-1275}.
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol and T. Sieminewska, Pure Appl. Chem. 57 (1985) 603.
R.W. Cranston and F.A. Inkley, Adv. Catal. 9 (1957) 143.
S. Lowell and J.E. Shields, Powder Surface Area and Porosity (Chapman & Hall, London, 1984).
E. Borgarello, J. Kiwi, M. Grätzel, I. Pelizzetti and M. Visca, J. Am. Chem. Soc. 104 (1982) 2996.
C.K. Jørgensen, Absorption Spectra and Chemical Bonding in Complexes (Pergamon Press, Oxford, 1962).
R.J. Capwell, F. Spagnolo and M.A. Sesa, Appl. Spectrosc. 26 (1972) 537.
G. Deo and I.E. Wachs, J. Phys. Chem. 95 (1991) 5889.
G. Ramis, G. Busca, C. Cristiani, A.S. Elmi and P. Villa, J. Mol. Catal. 61 (1990) 319.
D.S. Kim, M. Ostromecki and I.E. Wachs, J. Mol. Catal. 106 (1996) 93.
S.S. Chan, I.E. Wachs, L.L. Murrell, L. Wang and W.K. Hall, J. Phys. Chem. 88 (1984) 5831.
M.A. Vuurman, I.E. Wachs and A.M. Hit, J. Phys. Chem. 95 (1991) 9928.
C.F. Baes Jr. and R.E. Mesmer, The Hydrolysis of Cations (Wiley, New York, 1970).
I.R. Beattie and T.R. Gilson, J. Chem. Soc. (1965) 2322.
H. Miyata, Y. Nakagawa and H. Naguno, J. Chem. Soc. Faraday Trans. I 79 (1983) 2343.
V. Rives, Opt. Pura Apl. 16 (1983) 61.
L. Palmisano, V. Augugliaro, M. Schiavello and A. Sclafani, J. Mol. Catal. 56 (1989) 284.
V. Augugliaro, L. Palmisano, M. Schiavello, A. Sclafani, L. Marchese, G. Martra and F. Miano, Appl. Catal. 69 (1991) 323.
V. Augugliaro, M.J. López-Muñoz, L. Palmisano and J. Soria, Appl. Catal.A 101 (1993) 7.
I. Sopyan, M. Watanabe, S. Murasawa, K. Hashimoto and A. Fujishima, J. Photochem. Photobiol.A 98 (1996) 79.
A. Sclafani, L. Palmisano and M. Schiavello, J. Phys. Chem. 94 (1990) 829.
A.P. Rivera, K. Tanaka and T. Hisanaga, Appl. Catal. B 3 (1993) 37.
Rights and permissions
About this article
Cite this article
Martín, C., Solana, G., Rives, V. et al. Physico-chemical properties of WO3/TiO2 systems employed for 4-nitrophenol photodegradation in aqueous medium. Catalysis Letters 49, 235–243 (1997). https://doi.org/10.1023/A:1019025926206
Issue Date:
DOI: https://doi.org/10.1023/A:1019025926206