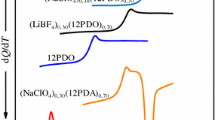
Abstract
Thermal analysis of aqueous solutions in which the solute does not crystallize immediately upon freezing was carried out to define the effects of experimental parameters on thermograms in the glass transition region. The intensity of enthalpy relaxations in the glass transition region is related to both the rate of cooling and the rate of heating through the glass transition region—slow cooling or slow heating increases the extent of structural relaxation in the glassy state and increases the intensity of the endotherm. Plots of the logarithm of heating rate versus l /Tg′ are linear, and activation enthalpies for structural relaxation are in the range of 210–350 kJ/mol. For polymeric solutes, both the activation enthalpies for structural relaxation and the heat capacity change accompanying the glass transition increase with increasing molecular weight of the solute. Molecular weight dependence of the observed midpoint of the glass transition agrees with the Fox–Flory relationship. Results are compared and contrasted with glass transitions in solid polymers and with the glass transition of hyperquenched water. Practical implications for characterization of formulations intended for freeze-drying are discussed.
Similar content being viewed by others
REFERENCES
F. Franks. Freeze drying: From empiricism to predictability. Cryo-Letters 11:93–110 (1990).
A. P. MacKenzie. Collapse during freeze drying-qualitative and quantitative aspects. In Goldblith, Rey, and Rothmayr (eds.), Freeze Drying and Advanced Food Technology, Academic Press, New York, 1975, pp. 277–307.
M. J. Pikal and S. Shah. The collapse temperature in freeze drying: Dependence on measurement methodology and rate of water removal from the glass phase. Int. J. Pharm. 62:165–186 (1990).
B. Wunderlich, D. M. Bodily, and M. H. Kaplan. Theory and measurements of the glass-transformation interval of polystyrene. J. Appl. Phys. 35:95–102 (1964).
M. L. Roy, M. J. Pikal, E. C. Rickard, and A. M. Maloney. The effects of formulation and moisture on the stability of a freeze-dried monoclonal antibody-vinca conjugate: A test of the WLF glass transition theory. Dev. Biol. Stand. 74:323–340 (1991).
I. M. Hodge. Effects of annealing and prior history on enthalpy relaxation in glassy polymers. 6. Adam-Gibbs formulation of nonlinearity. Macromolecules 20:2897–2908 (1987).
C. T. Moynihan, A. J. Easteal, J. Wilder and J. Tucker. Dependence of the glass transition temperature on heating and cooling rate. J. Phys. Chem. 2673–2677 (1974).
H. Levine and L. Slade. Thermomechanical properties of small carbohydrate-water glasses and “rubbers.” J. Chem. Soc. Faraday Trans. 84:2619–2633 (1988).
R. J. Bellows and C. J. King. Freeze drying of aqueous solutions: Maximum allowable operating temperature. Cryobiology 9:559–561 (1972).
A. P. MacKenzie. Physicochemical basis for the freeze drying process. Dev. Biol. Stand. 36:51–67 (1977).
G. P. Johari, A. Hallbrucker, and E. Mayer. Calorimetric relaxation and glass transition in poly(propylene glycols) and its monomer. J. Polym. Sci. Polym. Phys. 26:1923–1930 (1988).
A. Hallbrucker, E. Mayer, and G. P. Johari. The heat capacity and glass transition of hyperquenched glassy water. Philos. Mag. 60:179–187 (1989).
R. F. Boyer. CpTg and related quantities for high polymers. J. Macromol. Sci. Phys. B7:487–501 (1973).
J. Sasabe and C. T. Moynihan. Structural relaxation in poly(vinyl acetate). J. Polym. Sci. Polym. Phys. 16:1447–1457 (1978).
J. J. Aklonis and W. J. MacKnight. Introduction to Polymer Viscoelasticity, 2nd ed., John Wiley & Sons, New York, 1983, Chap. 4.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Her, LM., Nail, S.L. Measurement of Glass Transition Temperatures of Freeze-Concentrated Solutes by Differential Scanning Calorimetry. Pharm Res 11, 54–59 (1994). https://doi.org/10.1023/A:1018989509893
Issue Date:
DOI: https://doi.org/10.1023/A:1018989509893