Abstract
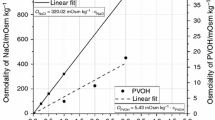
The effects of process and storage conditions of solid-state emulsions were studied. Oil-in-water emulsions may be prepared from solid state emulsions by adding an aqueous phase to the solid. Solid-state emulsions are prepared by processing an oil phase and an aqueous solution of matrix material via a solvent removal process. Sucrose, the carrier material utilized in this report, results in a metastable solid or glass, which can transform upon aging to a more stable thermodynamic state. Aging was determined by monitoring the crystallinity as a function of time, temperature, relative humidity, and grinding. The crystallinity of solid-state emulsions was determined with X-ray diffraction and differential scanning calorimetry. Results indicate that solid-state emulsions should be stored between 15 and 25% relative humidity at 25°C. Grinding has no apparent effect on the crystallinity of the sample, as detected by X-ray diffraction, although the microcrystallinity is increased. The utilization of silinized glassware enabled the sample-to-sample microcrystalline variability to be reduced.
Similar content being viewed by others
REFERENCES
W. L. Chiou and S. Reigelman. Pharmaceutical applications of solid dispersion systems. J. Pharm Sci. 60:1281–1302 (1971).
L. V. Allen, V. A. Yanchick, and D. D. Maness. Dissolution rates of corticosteroids utilizing sugar glass dispersions. J. Pharm. Sci. 66:494–487 (1977).
W. L. Chiou and S. Riegelman. Preparation and dissolution characteristics of several fast-release solid dispersions of griseofulvin. J. Pharm. Sci. 58:1505–1509 (1969).
J. L. Ford. The current status of solid dispersions. Pharm. Acta Helv. 61:69–88 (1986).
R. J. Timko and N. C. Lordi. Thermal characterization of citric acid solid dispersions with benzoic acid and phenobarbital. J. Pharm. Sci. 68:601–605 (1979).
J. L. Ford. The use of thermal analysis in the study of solid dispersions. Drug Dev. Ind. Pharm. 13:1741–1777 (1987).
E. I. Stupak and T. R. Bates. Enhanced absorption and dissolution of reserpine from reserpine-polyvinylpyrrolidone coprecipitates. J. Pharm. Sci. 61:400–403 (1972).
A. Halpern and C. H. Bradney. U.S. Patent 2,698,822 (1955).
A. Thakkar, C. A. Hirsch, and J. G. Page. Solid dispersion approach for overcoming bioavailability problems due to polymorphism of nabilone, a cannabinoid derivative. J. Pharm. Pharmacol. 29:783–784 (1977).
H. P. Merkle. Aging of coprecipitates: Self-association as observed by dynamic dialysis of supersaturated aqueous solutions of hydrocortisone. Pharm. Acta Helv. 57:1601–1633 (1982).
R. J. Timko and N. G. Lordi. Thermal analysis studies of glass dispersion systems. Drug Dev. Ind. Pharm. 10:425–451 (1984).
I. Sugimoto, A. Kuchiki, H. Nakagawa, K. Tohgo, S. Kondo, and K. Takahashi. Dissolution and absorption of nifedipine from nifedipine-coprecipitate. Drug Dev. Ind. Pharm. 6:137–160 (1980).
S. L. Myers and M. L. Shively. Preparation and characterization of emulsifiable glasses: Oil-in-water and water-in-oil-in-water emulsions. J. Colloid Interface Sci. 149:271–278 (1992).
M. Mathiouthi. Physical state of sucrose after lyophilization. II. Ind. Aliment. Agr. 92:1279–1285 (1975).
A. Smith. Use of thermal analysis in predicting drug-excipient interactions. Anal. Proc. 19:559–561 (1982).
N. A. Peppas and E. W. Merrill. Differential scanning calorimetry of crystallized PVA hydrogels. J. Appl. Polym. Sci. 20:1457–1465 (1976).
J. T. Carstensen and K. Van Scoik. Amorphous-to-crystalline transformation of sucrose. Pharm. Res. 7:1278–1281 (1990).
K. Van Scoik. Nucleation and Crystallization Phenomena in Amorphous Sucrose Systems, Ph.D. thesis, School of Pharmacy, University of Wisconsin, Madison, 1987, pp. 167–176.
D. R. Uhlmann and B. Chalmers. The energetics of nucleation. In Nucleation Phenomena, American Chemical Society, Washington, DC, 1966.
S. K. Heffels, L. J. Kuijvenhoven, and E. J. de Jong. In S. J. Jancis and E. J. de Jong (eds.), Industrial Crystallization 84, Elsevier Science, Amsterdam, 1984.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shively, M.L., Myers, S. Solid-State Emulsions: The Effects of Process and Storage Conditions. Pharm Res 10, 1071–1075 (1993). https://doi.org/10.1023/A:1018983227234
Issue Date:
DOI: https://doi.org/10.1023/A:1018983227234