Abstract
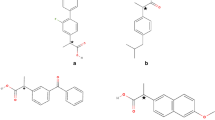
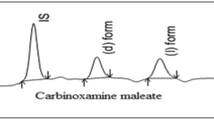
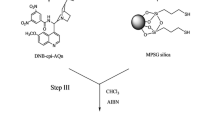
Several approaches to the separation of four stereoisomers, 1–4, of a novel, topically active, carbonic anhydrase inhibitor, 1, with two chiral centers in the molecule and four isomers, 5–8, of its chiral metabolite, 5, were evaluated. These methods include nonchiral derivatization followed by separation on chiral stationary phases (CSPs) and chiral derivatization and separation on nonchiral columns and on CSPs. Baseline separation of stereoisomers 1–4 was achieved in less than 15 min after chiral derivatization with (S)-(+)-l-(l-naphthyl)ethyl isocyanate (NEIC) and chiral chromatography on a (R)-N-(3,5-dinitrobenzoyl)phenyl glycine (DNBPG) column under normal phase (NP) conditions. Similarly, isomers 5-8 were baseline separated in less than 20 min after derivatization with NEIC and chromatography on nonchiral (nitrophenyl) and chiral [(S)-(3,5-dinitrobenzoyl)leucine; DNBL] columns in series under the same NP chromatographic conditions. Only partial separation of the diastereomeric derivatives was observed on a variety of nonchiral columns. In addition, all other direct and indirect chiral separation approaches gave only partial separation of at least two stereoisomers within the group of 1–4 or 5–8. The details of chiral separations using various methods and separation (α) and capacity factors (k′) of the derivatized isomers 1–8 on a series of chiral and nonchiral columns are presented. Using these methods, the absolute configuration of the human metabolite of 1 was established as S 1 S 2 (5), and the heat (HD) and light (LD) degradation products of 1 as R 1 S 2 (3) and S1 S 2 (5), respectively.
Similar content being viewed by others
REFERENCES
J. J. Baldwin, G. S. Ponticello, P. S. Anderson, M. A. Mercko, W. C. Randall, H. Schwam, M. F. Sugrue, P. S. Gautheron, J. Grove, P. Mallorga, M.-P. Viader, B. M. McKeever, and M. A. Navia. Thienothiopyran-2-sulfonamides: Novel topically active carbonic anhydrase inhibitors for the treatment of glaucoma. J. Med. Chem. 32:2513–2518 (1989).
T. J. Blacklock, P. Sohar, J. W. Butcher, T. Lamanec, and E. J. J. Grabowski. An enantioselective synthesis of the topically-active carbonic anhydrase inhibitor MK-507; 5,6-dihydro-4H-(S)-4-ethylamino-(S)-6-methylthieno[2,3-b]-thiopyran-2-sulfonamide-7,7-dioxide hydrochloride. J. Org. Chem. 58:1672–1679 (1993).
B. K. Matuszewski and M. L. Constanzer. Indirect chiral separation and analyses in biological fluids of the stereoisomers of a thienothiopyran-2-sulfonamide (Trusopt), a novel carbonic anhydrase inhibitor with two chiral centers in the molecule. Chirality 4:515–519 (1992).
W. Lindner and C. Pettersson. Resolution of optical isomers by liquid chromatographic techniques. In I. Wainer (ed.), Liquid Chromatography in Pharmaceutical Development, Aster, Springfield, OR, 1986, pp. 63–131.
L. E. Edholm, C. Lindberg, J. Paulson, and A. Walhagen. Determination of drug enantiomers in biological samples by coupled column liquid chromatography and liquid chromatography-mass spectrometry. J. Chromatogr. (Biomed. Appl.) 424:61–72 (1988).
J. J. Baldwin, G. S. Ponticello, and M. F. Sugrue. MK-507. Drugs Future 15:350–351 (1990).
P. Quint. Unpublished results.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matuszewski, B.K., Constanzer, M.L. & Kiganda, M. Analytical Chiral Separation of the Stereoisomers of a Novel Carbonic Anhydrase Inhibitor and Its Deethylated Metabolite, and the Assignment of Absolute Configuration of the Human Metabolite and Chiral Degradation Products. Pharm Res 11, 449–454 (1994). https://doi.org/10.1023/A:1018981524856
Issue Date:
DOI: https://doi.org/10.1023/A:1018981524856