Abstract
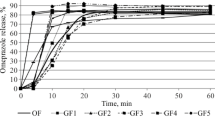
The bioavailability of three marketed controlled-release dosage forms and a reference solution of theophylline was studied in eight subjects with normal gastric fluid acidity and seven subjects who were achlorhydric. Gastric pH was monitored with a Heidelberg capsule. One of the controlled-release dosage forms dissolved more rapidly in vitro when exposed to acid conditions, one dissolved more rapidly in pH 7.5 media, and the third dissolved at a rate independent of pH. Using a crossover design, each subject received each dosage form twice. Blood was sampled for up to 47 hr after each dose, and serum was assayed for theophylline by HPLC. The product which dissolved more rapidly under acid conditions in vitro exhibited a 3 hr longer T max in the achlorhydrics compared to the normal subjects. The product which dissolved more rapidly in the pH 7.5 media exhibited a relatively higher AUC(0–∞) in the achlorhydric subjects than in normal subjects after the AUC data were normalized for clearance differences between the two subject groups. The in vivo bioavailability of these dosage forms could be related to the in vitro dissolution characteristics for some parameters. However, with the exception of the mean T max values, the mean bioavailability parameters differed by less than 20% between the two subject groups.
Similar content being viewed by others
REFERENCES
H. Ogata, N. Aoyagi, N. Kaniwa, M. Koibuchi, T. Shibazaki, A. Ejima, S. Tsuji, and Y. Kawazu. Bioavailability of diazepam from uncoated tablets in humans. Its correlation with the dissolution rates of the tablets. Int. J. Clin. Pharmacol. Ther. Toxicol. 20:159–165 (1982).
H. Ogata, N. Aoyagi, N. Kaniwa, M. Koibuchi, T. Shibazaki, A. Ejima, S. Tsuji, and Y. Kawazu. Bioavailability of diazepam from uncoated tablets in humans. Effect of gastric fluid acidity. Int. J. Clin. Pharmacol. Ther. Toxicol. 20:166–170 (1982).
V. K. Prasad, V. P. Shah, P. Knight, H. Malinowski, B. E. Cabana, and M. C. Meyer. Importance of media selection in establishment of in vitro-in vivo relationships for quinidine gluconate. Int. J. Pharm. 13:1–7 (1983).
R. R. Berardi, J. B. Dressman, G. H. Elta, and G. J. Szpunar. Elevation of gastric pH with ranitidine does not affect the release characteristics of sustained release ibuprofen tablets. Biopharm Drug Dispos. 9:337–347 (1988).
V. I. Vashi and M. C. Meyer. The effect of pH on the in vitro dissolution and the in vivo absorption of controlled-release theophylline in dogs. J. Pharm. Sci. 77(9):760–764 (1988).
P. M. Christiansen. The incidence of achlorhydria and hypochlorhydria in healthy subjects and patients with diseases. Scand. J. Gastroent. 3:497–508 (1968).
M. Bins, P. I. C. J. Burgers, S. G. M. Selbach, Th. B. van Wettum, C. B. H. W. Lamers, and J. H. M. van Tongeren. Prevalence of achlorhydria in a normal population and its relation to serum gastrin. Hepato. Gastroenrol. 31:41–43 (1984).
Heidelberg pH Capsule System, Training and Instruction Manual, 5th ed., Heidelberg International, Norcross, GA, 1985.
A. M. Connell and T. E. Waters. Assessment of gastric function by pH telemetering capsule. Lancet 2:227–230 (1964).
W. C. Watson and E. Paton. Studies on intestinal pH by radiotelemetering. Gut 6:606–612 (1965).
D. R. Yarbrough, J. C. McAlhany, N. Cooper, and M. G. Weidner. Evaluation of the Heidelberg pH capsule. Am. J. Surg. 117:185–192 (1969).
J. B. Dressman and G. L. Amidon. Radiotelemetric method for evaluating enteric coatings in vivo. J. Pharm. Sci. 73:935–938 (1984).
C. Y. Lui, G. L. Amidon, R. R. Berardi, D. Fleisher, C. Youngberg, and J. B. Dressman. Comparison of gastrointestinal pH in dogs and humans: Implications on the use of the beagle dog as a model for oral absorption in humans. J. Pharm. Sci. 75:271–273 (1986).
M. M. Wolfe and A. H. Soll. The physiology of gastric acid secretion. N. Engl. J. Med. 319(26):1707–1715 (1988).
H. N. Farrish and W. A. Wargin. Separation and quantitation of theophylline and paraxanthine by reversed-phase liquid chromatography. Clin. Chem. 26:524–525 (1980).
J. Wagner. Fundamentals of Clinical Pharmacokinetics, Drug Intelligence, Hamilton, IL, 1975, p. 175.
K. S. Albert and R. B. Smith, In K. S. Albert (ed.), Drug Absorption and Disposition: Statistical Considerations, American Pharmaceutical Association, Washington, DC, 1980, pp. 87–114.
A. Karim. Theophylline with food: Theo-24. Am. Pharm. NS25:132–133 (1985).
R. Penagini, R. C. Spiller, J. J. Misiewicz, P. G. Frost, and D. B. A. Silk. Effect of cholecystectomy on mouth-to-cecum transit of a liquid meal. Digest Dis. Sci. 33(1):19–22 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meyer, M.C., Straughn, A.B., Jarvi, E.J. et al. The Effect of Gastric pH on the Absorption of Controlled-Release Theophylline Dosage Forms in Humans. Pharm Res 10, 1037–1045 (1993). https://doi.org/10.1023/A:1018923008579
Issue Date:
DOI: https://doi.org/10.1023/A:1018923008579