Abstract
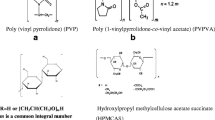
Purpose. To measure solid-state features of amorphous molecular dispersions of indomethacin and various molecular weight grades of poly(vinylpyrrolidone), PVP, and poly(vinylpyrrolidone-co-vinylacetate), PVP/VA, in relation to isothermal crystallization of indomethacin at 30°C
Methods. The glass transition temperatures (Tg) of molecular dispersions were measured using differential scanning calorimetry (DSC). FT-IR spectroscopy was used to investigate possible differences in interactions between indomethacin and polymer in the various dispersions. The enthalpy relaxation of 5% w/w and 30% w/w polymer dispersions was determined following various aging times. Quantitative isothermal crystallization studies were carried out with pure indomethacin and 5% w/w polymers in drug as physical mixtures and molecular dispersions.
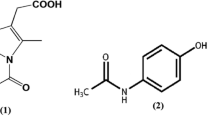
Results. All coprecipitated mixtures exhibited a single glass transition temperature. All polymers interacted with indomethacin in the solid state through hydrogen bonding and in the process eliminated the hydrogen bonding associated with the carboxylic acid dimers of indomethacin. Molecular mobility at 16.5°C below Tg was reduced relative to indomethacin alone, at the 5% w/w and 30% w/w polymer level. No crystallization of indomethacin at 30°C was observed in any of the 5% w/w polymer molecular dispersions over a period of 20 weeks. Indomethacin alone and in physical mixtures with various polymers completely crystallized to theγ form at this level within 2 weeks.
Conclusions. The major basis for crystal inhibition of indomethacin at 30°C at the 5% w/w polymer level in molecular dispersions is not related to polymer molecular weight and to the glass transition temperature, and is more likely related to the ability to hydrogen bond with indomethacin and to inhibit the formation of carboxylic acid dimers that are required for nucleation and growth to the γ crystal form of indomethacin.
Similar content being viewed by others
REFERENCES
K. Yamamoto, M. Nakano, T. Arita, Y. Takayama, and Y. Nakai. Dissolution behavior and bioavailability of phenytoin from a ground mixture with microcrystalline cellulose. J. Pharm. Sci. 65:1484–1488 (1970).
W. L. Chiou and S. T. Reigelman. Oral absorption of griseofulvin in dogs. Increased absorption via solid dispersion in polyethylene glycol 6000. J. Pharm. Sci. 59:937–942 (1970).
H. Imaizumi, N. Nambu, and T. Nagai. Stability and several physical properties of amorphous and crystalline forms of indomethacin. Chem. Pharm. Bull. 28:2565–2569 (1980).
M. Otsuka and N. Kaneniwa. A kinetic study of the crystallization process of noncrystalline indomethacin under isothermal conditions. Chem. Pharm. Bull. 36:4026–4032 (1988).
V. Andronis, M. Yoshioka, and G. Zografi. Effect of sorbed water on the crystallization of indomethacin from the amorphous state. J. Pharm. Sci. 86:346–351 (1997).
W. L. Chiou and S. T. Reigelman. Pharmaceutical application of solid dispersion systems. J. Pharm. Sci. 60:1281–1302 (1971).
A. P. Simonelli, S. C. Mehta, and W. I. Higuchi. Dissolution rates of higher energy sulfathiazole-povidone coprecipitates 2: Characterization of form of drug controlling its dissolution rate via solubility studies. J. Pharm. Sci. 65:355–361 (1976).
H. Imaizumi, N. Nambu, and T. Nagai. Stabilization of amorphous state of indomethacin by solid dispersion in polyvinylpyrrolidone. Chem. Pharm. Bull. 31:2510–2512 (1983).
H. Sekizaki, K. Danjo, H. Eguchi, Y. Yonezawa, H. Sunada, and A. Otsuka. Solid-state interaction of ibuprofen with polyvinylpyrrolidone. Chem. Pharm. Bull. 43:988–993 (1995).
M. Yoshioka, B. C. Hancock, and G. Zografi. Crystallization of indomethacin from the amorphous state below and above its glass transition temperature. J. Pharm. Sci. 83:1700–1705 (1994).
V. Andronis and G. Zografi. The molecular mobility of supercooled amorphous indomethacin as a function of temperature and relative humidity. Pharm. Res. 15:835–842 (1998).
C. A. Okasanen and G. Zografi. The relationship between the glass transition temperature and water vapor absorption by poly(-vinylpyrrolidone). Pharm. Res. 7:654–657 (1990).
C. A. Okasanen and G. Zografi. Molecular mobility in mixtures of absorbed water and solid poly(vinylpyrrolidone). Pharm. Res. 10:791–799 (1993).
M. Yoshioka, B. C. Hancock, and G. Zografi. Inhibition of indomethacin crystallization in poly(vinylpyrrolidone) coprecipitates. J. Pharm. Sci. 84:983–986 (1995).
L. S. Taylor and G. Zografi. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm. Res. 14:1691–1698 (1997).
V. Andronis. Crystallization of amorphous indomethacin. Ph. D. Thesis. University of Wisconsin-Madison (1997).
V. Buhler. Kollidone, Second ed., BASF Akiengesellschaft, Ludwigshafen, 1993.
B. C. Hancock, S. L. Shamblin, and G. Zografi. The molecular mobility of amorphous pharmaceutical solids below their glass transition temperature. Pharm. Res. 12:799–806 (1995).
S. L. Shamblin and G. Zografi. Enthalpy relaxation in binary amorphous mixtures containing sucrose. Pharm. Res. 15:1828–1834 (1998).
B. C. Hancock, C. R. Dalton, M. J. Pikal, and S. L. Shamblin. A pragmatic test of a simple calorimetric method for determining the fragility of some amorphous pharmceutical materials. Pharm. Res. 15:762–767 (1998).
R. Simha and R. F. Boyer. On a general relation involving the glass temperature and coefficients of expansion of polymers. J. Chem. Phys. 37:1003–1007 (1962).
M. Gordon and J. S. Taylor. Ideal copolymers and the second-order transition of synthetic rubbers 1. Non-crystalline co-polymers. J. Appl. Chem. 2:493–500 (1952).
P. R. Couchman and F. E. Karasz. A classical thermodynamic discussion on the effect of composition on glass-transition temperatures. Macromol. 11:117–119 (1978).
S. L. Shamblin, L. S. Taylor, and G. Zografi. Mixing behavior of colyophilized binary systems. J. Pharm. Sci. 87:694–701 (1998).
J. Cowie and R. Ferguson. Physical aging studies in poly(vinyl methyl ether). 1. Enthalpy relaxation as a function of aging temperature. Macromol. 22:2307–2312 (1989).
I. M. Hodge. Enthalpy relaxation and recovery in amorphous materials. J. Non-Cryst. Solids 169:211–266 (1994).
A. Brunacci, J. Cowie, R. Ferguson, and I. McEwen. Enthalpy relaxation in glassy polystyrenes: 1. Polymer 38:865–870 (1997).
M. D. Ediger, C. A. Angell, and S. R. Nagel. Supercooled liquids and glasses. J. Phys. Chem. 100:13200–13212 (1996).
T. J. Kistenmacher and R. E. March. Crystal and molecular structure of an antiinflammatory agent. Indomethacin. J. Amer. Chem. Soc. 94:1340–1345 (1972).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, T., Zografi, G. Physical Properties of Solid Molecular Dispersions of Indomethacin with Poly(vinylpyrrolidone) and Poly(vinylpyrrolidone-co-vinyl-acetate) in Relation to Indomethacin Crystallization. Pharm Res 16, 1722–1728 (1999). https://doi.org/10.1023/A:1018906132279
Issue Date:
DOI: https://doi.org/10.1023/A:1018906132279