Abstract
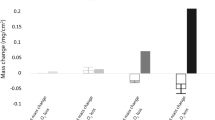
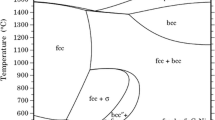
The corrosion of Co-15 wt.% Y has been studiedat 600-800°C inH2-H2S-CO2 mixturesproviding a sulfur pressure of 10-8 atm at600-800°C and of 10-7 atm at 800°Cand an oxygen pressure of 10-24 atm at 600°C and of10-20 atm at 700-800°C. The corrosionrates in such sulfidizing-oxidizing atmospheres werecompared with those of pure cobalt and yttrium. Theaddition of yttrium to cobalt is only slightly beneficial, sincefor a yttrium content of 15 wt.% the corrosion rate isreduced quite significantly with respect to pure cobaltat 800°C under 10-7 atm S2,only to a limited extent at 600°C, and even slightlyincreased at 700°C. Moreover, the alloy corrodesconsiderably more rapidly than pure yttrium at800°C, when the latter behaves protectively. At 600 and 700°C, yttrium exhibitedbreakaway behavior, while the alloy corroded morerapidly than yttrium at short times, but more slowly atlong times. Under all conditions, except at 800°Cunder 10-8 atm S2, the alloy formsan external layer of cobalt sulfide overlying anintermediate region of very complex compositioncontaining a mixture of the compounds of the two metalsand an innermost region of internal attack containing compoundsof yttrium with both oxygen and sulfur. Thus, cobalt canstill diffuse through the intermediate region to formthe outer cobalt-sulfide layer at nonnegligible rates. The scaling behavior of the Co-15% Yalloy is discussed by taking into account the limitedsolubility of yttrium in cobalt as well as the presenceof an intermetallic Co-Y compound in thealloy.
Similar content being viewed by others
REFERENCES
K. Natesan, Corrosion 41, 646 (1985).
F. Gesmundo, D. J. Young, and S. K. Roy, High Temp. Mater. Proc. 8, 149 (1989).
J. Stringer, in High-Temperature Oxidation and Sulfidation Processes, J. D. Embury, ed. (Pergamon Press, New York, 1990), p. 257.
F. Gesmundo, in High Temperature Materials for Power Engineering 1990, Vol. I, E. Bachelet, ed. (Kluwer Academic Publishers, Dordrecht, 1990), p. 67.
G. Y. Lai, High-Temperature Corrosion of Engineering Alloys (ASM International, Materials Park, Ohio, USA, 1990).
S. Mrowec and K. Przybylski, High Temp. Mater. Proc. 6, 1 (1984).
B. Gleeson, D. L. Douglass, and F. Gesmundo, Oxid. Met. 31, 209 (1989).
M. F. Chen, D. L. Douglass, and F. Gesmundo, Oxid. Met. 31, 237 (1989).
R. V. Carter, D. L. Douglass, and F. Gesmundo, Oxid. Met. 31, 341 (1989).
M. F. Chen and D. L. Douglass, Oxid. Met. 32, 185 (1989).
G. Wang, R. Carter, and D. L. Douglass, Oxid. Met. 32, 273 (1989).
B. Gleeson, D. L. Douglass, and F. Gesmundo, Oxid. Met. 33, 425 (1990).
Y. Niu, F. Gesmundo, and F. Viani, Corros. Sci. 36, 423 (1994).
Y. Niu, F. Gesmundo, and F. Viani, Corros. Sci. 36, 853 (1994).
Y. Niu, F. Gesmundo, and F. Viani, Corros. Sci. 36, 883 (1994).
Y. Niu, F. Gesmundo, C. L. Zeng, W. T. Wu, and F. Viani, Oxid. Met. 48, 243 (1997).
K. N. Strafford, G. R. Winstanley, and J. M. Harrison, Werkst. Korros. 25, 487 (1974).
C. N. R. Rao and K. P. R. Pisharody, Progress in Solid State Chemistry, Vol. X, J. O. McCaldin and G. Somorjai, eds. (Pergamon Press, New York, 1975), p. 207.
Y. Niu, R. Y. Yan, W. T. Wu, and F. Gesmundo, Corros. Sci., 39, 1831 (1997).
T. B. Massalski, ed., Binary Alloy Phase Diagrams (ASM, Materials Park, Ohio, USA, 1990).
Y. Niu, F. Gesmundo, and F. Viani, Corros. Sci. 36, 1973 (1994).
A. Rahmel, M. Schorr, A. Velasco-Tellez, and A. Pelton, Oxid. Met. 27, 199 (1987).
K. C. Mills, Thermodynamic Data for Inorganic Sulphides, Selenides and Tellurides (Butterworth, London, 1974).
F. Gesmundo, F. Viani, and Y. Niu, Oxid. Met. 42, 409 (1994).
F. Gesmundo, F. Viani, and Y. Niu, Oxid. Met. 43, 379 (1994).
A. Meijering, in Advances in Materials Science, H. Herman, ed. (Wiley Interscience, New York, 1971), p. 1.
F. Gesmundo and Y. Niu, Oxid. Met. 51, 131 (1999).
D. J. Young and S. Watson, Oxid. Met. 44, 239 (1995).
C. Wagner, Z. Elektrochem. 63, 772 (1959).
R. A. Rapp, Corrosion 21, 382 (1965).
F. Gesmundo, F. Viani, and Y. Niu, Oxid. Met. 45, 51 (1996).
F. Gesmundo, F. Viani, and Y. Niu, Oxid. Met. 47, 355 (1997).
F. Gesmundo, F. Viani, Y. Niu, and D. L. Douglass, Oxid. Met. 40, 373 (1993).
F. Gesmundo, F. Viani, Y. Niu, and D. L. Douglass, Oxid. Met. 42, 465 (1994).
Rights and permissions
About this article
Cite this article
Niu, Y., Gesmundo, F. & Li, Y.S. The Corrosion of Co-15 wt.% Y at 600-800°C in Sulfidizing-Oxidizing Atmospheres. Oxidation of Metals 51, 421–447 (1999). https://doi.org/10.1023/A:1018887110251
Issue Date:
DOI: https://doi.org/10.1023/A:1018887110251