Abstract
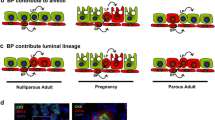
Progesterone was identified as a mammogenichormone several years ago but until now its precise rolein mammary development has remained obscure. Recentlywith the generation of several transgenic mouse models and development of reagents for analysisof progesterone receptor expression, the role ofprogesterone signaling in mammary development isbecoming more clear. The most significant observationsto emerge from these studies are (1) progesteronereceptors (PR)4 are present in a heterogeneous manner inthe epithelial cells and undetectable in the surroundingfat pad; (2) they are essential for lobuloalveolar and not for ductal morphogenesis; (3)progesterone signaling through progesterone receptors,leading to lobuloalveolar development, is initiated inthe epithelium and may occur through paracrinemechanisms; and (4) a regulated expression of the twoisoforms of progesterone receptor is critical formaintaining appropriate responsiveness to progesteroneand hence, epithelial cell replicative homeostasis.These studies also reveal that the consequences ofprogesterone signaling through progesterone receptor maydepend on the cell context, cell-cell andcell-extracellular matrix interactions, the dynamics ofPR turnover and the fate of PR positivecells.
Similar content being viewed by others
REFERENCES
D.C. Fernig and J.A. Smith (1991). Regulatory mechanisms in breast cancer. In M. Lippmann and R. Dickson (eds.), Cancer Treatment and Research, Kluwer Academic Press, pp. 47–77.
R.D. Cardiff (1998). Are the TDLU of the human the same as the LA of mice? J. Mam. Gland Biol. Neoplasia 3:3–5.
S. Nandi (1958). Endocrine control of mammary gland development and function in C3H/Crg1 mouse. J. Natl. Cancer Inst. 21:1039–1063.
Y.S. Topper and C.S. Freeman (1980). Multiple hormone interactions in the developmental biology of the mammary gland. Physiol. Rev. 60:1049.
C.W. Daniel and G.B. Silberstein (1987). Postnatal development of the rodent mammary gland. In M.C. Neville, C.W. Daniel (eds.), The Mammary Gland: Development, Regulation and Function, Plenum Press, New York, pp. 3–36.
S. Nandi, R.C. Gazman, and J.C. Yang (1995). Hormones and mammary carcinogenesis in mice, rats and humans: A unifying hypothesis. Proc. Natl. Acad. Sci. U.S.A. 92:3650–3657.
F. Bresciani (1968). Topography of DNA synthesis in mammary gland of the C3H mouse and its control by ovarian hormones: An autoradiographic study. Cell Tissue Kinetics 1:51–63.
C.W. Daniel, G.R. Silberstein, and P. Strickland (1987). Direct action of 17–beta estradiol on mouse mammary ducts analysed by sustained release implants and steroid autoradiography. Cancer Res. 47:6052–6057.
G.B. Silberstein, K. Van Horn, G. Shyamala, and C.W. Daniel (1994). Essential role of endogenous estrogen in directly stimulating mammary growth demonstrated by implants containing pure antiestrogens. Endocrinology 134:84–90.
R.R. Ichinose and S. Nandi (1966). Influence of hormones in lobulo alveolar differentiation of mouse mammary glands in vitro. J. Endocrinol. 35:331–340.
J. Russo and I.H. Russo (1995). The etiopathogenesis of breast cancer prevention. Cancer Lett. 90:81–89.
J. Russo and I.H. Russo (1996). Experimentally induced mammary tumors in rats. Breast Cancer Res. Treat. 39:7–20.
D. J. Mangelsdorf, C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, and R.M. Evans (1995). The nuclear receptor superfamily: The second decade. Cell 83:835–839.
M.J. Tsai and B.W. O'Malley (1994). Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann. Rev. Biochem. 63:451–486.
G. Shyamala, W. Schneider, and D. Schott (1990). Developmental regulation of murine mammary progesterone receptor gene expression. Endocrinology 126:2882.
J.P. Lydon, F.J. DeMayo, C.R. Funk, S.K. Mani, A.R. Hughes, C.A. Montgomery, Jr., G. Shyamala, O.M. Conneely, and B.W. O'Malley (1995). Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9:2266–2278.
W.P. Bocchinfuso and K.S. Korach (1997). Mammary gland development and tummorigenesis in estrogen receptor knockout mice. J. Mam. Gland Biol. Neoplasia 2:323–334.
J.M. Williams and C.W. Daniel (1983). Mammary ductal elongation: Differentiation of myo-epithelium and basal lamina during branching morphogenesis. Dev. Biol. 97:274–290.
D.R. Schott, G. Shyamala, W. Schneider, and G. Parry (1991). Molecular cloning, sequence analyses and expression of complementary DNA encoding murine progesterone receptor. Biochemistry 30:7014–7020.
G. Shyamala, MH. Barcellos-Hoff, D. Toft, and X. Yang (1997). In situ localization of progesterone receptors in normal mouse mammary glands: Absence of receptors in connective and adipose stroma and heterogeneous distribution in the epithelium. J. Steroid Biochem. Mol. Biol. 63:251–259.
G.B. Silberstein, K. Van Horn, G. Shyamala, and C.W. Daniel (1996). Progesterone receptors in the mouse mammary duct: Distribution and developmental regulation. Cell Growth Differ. 7:975–952.
S. Z. Haslam and G. Shyamala (1981). Relative distribution of estrogen and progesterone receptors among the epithelial, adipose and connective tissue components of the mammary gland. Endocrinology 108:825–830.
W. Stumpf and M. Sar (1976). Autoradiographic localization of estrogen, androgen, progestin, and glucocorticosteroid in “target tissues” and “nontarget tissues.” In J. Pasqualini (ed.), Receptors and Mechanisms of Action of Steroid Hormones, Marcel Dekker Inc., New York, pp. 41–84.
M. Warembourg (1983). Progestagen-concentrating cells in the brain, uterus, vagina and mammary glands of galago (Galago senegalensis). J. Reprod. Fertil. 68:189–193.
M.F. Press and G.L. Greene (1988). Localization of progesterone receptor with monoclonal antibodies to human progestin receptor. Endocrinology 122:1165–1175.
C. Brisken, S. Park, T. Vass, J.P. Lydon, B.W. O'Malley, and R.A. Weinberg (1998). A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. U.S.A. 95:5076–5081.
G. Shyamala (1997). Roles of estrogen and progesterone in normal mammary gland development: Insights from progesterone receptor null mutant mice and in situ localization of receptor. Trends Endocrinol. Metabol. 8(1):34–39.
E. Anderson, R.B. Clarke, and A. Howell (1998). Estrogen responsiveness and control of normal human breast proliferation. J. Mam. Gland Biol. Neoplasia 3:37–48.
G.H. Smith and D.A. Medina (1988). A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J. Cell. Sci. 89:173–183.
G.H. Smith (1996). Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res. Treat. 38:21–31.
G. Chepko and G.H. Smith (1997). Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29:239–253.
W. Schneider, C. Ramachandran, P.G. Satyaswaroop, and G. Shyamala (1991). Murine progesterone receptor exists predominantly as the 83–kilodalton “A” form. J. Steroid Biochem. Mol. Biol. 38:285–291.
E. Vegeto, M.M. Shahbaz, D.X. Wen, M.E. Goldman, B.W. O'Malley, and D.P. McDonnell (1993). Human progesterone receptor A form is a cell-and promoter-specific repressor of human progesterone receptor B function. Mol. Endocrinol. 7(10):1244–1255.
D.P. McDonnell (1995). Unraveling the human progesterone receptor signal transduction pathway: Insights into anti-progestin action. Trends Endocrinol. Metabol. 6:133–138.
G. Shamala, X. Yang, G.B. Silberstein, M.H. Barcellos-Hoff, and E. Dale (1998). Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc. Natl. Acad. Sci. U.S.A. 95:696–701.
C.W. Daniel, P. Strickland, and Y. Friedmann (1995). Expression and functional role of E-and P-cadherins in mouse mammary ductal morphogenesis and growth. Dev. Biol. 169(2):511–519.
B. D'souza and J. Taylor-Papadimitriou (1994). Overexpression of ERBB2 inhuman mammary epithelial cells signals inhibition of transcription of the E-cadherin gene. Proc. Natl. Acad. Sci. U.S.A. 91(15):7202–7206.
M. Takeichi (1988). The cadherins: Cell-cell adhesion molecules controlling animal morphogenesis. Development 102(4):639–655.
M.J. Warburton, D. Mitchell, E.J. Ormerod, and P. Rudland (1982). Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J. Histochem. Cytochem. 30:667–676.
M.S. Wicha, L.A. Liotta, B.K. Vonderhaar, and W.R. Kidwell (1980). Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Dev. Biol. 80:253–256.
C.J. Sympson, M.J. Bissell, and Z. Werb (1995). Mammary gland tumor formation in transgenic mice overexpressing stromelysin-1. Sem. Cancer Biol. 6:159–163.
D.J.P. Ferguson and J.J. Anderson (1981). Morphological evaluation of cell turnover in relation to the menstural cycle in the “resting” human breast. Brit. J. Cancer 44:177–181.
J.J. Going, J.J. Anderson, S. Battersby, and C.C.A. Macintyre (1988). Proliferative and secretory activity of human breast during natural and artificial menstrual cycles. Am. J. Pathol. 130:193–204.
J. E. Ferguson, A.M. Schor, A. Howell, and M.W. Ferguson (1992). Changes in the extracellular matrix of the normal human breast during the menstrual cycle. Cell Tissue Res. 268:167–177.
K.B. DeOme, L.J. Faulkin, H.A. Bern, and P.B. Blair (1959). Development of mamamry tumors from hyperplastic alveolar nodules transplanted into gland-free mamamry fat pads of female C3H mice. Cancer Res. 19:515.
S. Coleman, G.b. Silberstein, and C.W. Daniel (1988). Ductal morphogensis in the mouse mammary gland: Evidence supporting a role for epidermal growth factor. Dev. Biol. 127:304–315.
J.D. Graham and C.L. Clarke (1997). Physiological action of progesterone in target tissues Endocrine Rev. 18:502–519.
J.I. Emerman and D.R. Pitelka (1977). Maintenance and induction of morphological differentiation in dissociated mammary epithelial cells by floating collagen membranes. In vitro 13:316–328.
M.H. Barcellos-Hoff and M.J. Bissell (1989). Mammary epithelial cells as a model for studies of the regulation of gene expression. In A.R. Liss (ed.), Functional Epithelial Cells in Culture, Alan R. Liss, Inc., pp. 399–433.
S.Z. Haslam and M.L. Levely (1985). Estrogen responsiveness of normal mouse mammary cells in primary cell culture: Association of mammary fibroblasts with estrogenic regulation of PgR. Endocrinology 116:1835–1844
J. Rubin, H. Osada, P. Finch, W. Taylor, S. Rudikoff, and S. Aaronson (1989). Purification and characterization of newly identified growth factor specific for mammary epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 86:802–806.
T. Ulich, E. Yi, R. Cardiff, S. Yin, N. Bikhaze, R. Biltz, C. Morris, and G. Pierce (1994). Keratinocyte growth factor for mammary epithelium in vivo. Am. J. Pathol. 144:862–868.
S. Wilson, J. Weng, E. Chwang, L. Gollahan, A. Leitch, and J. Shay (1994). Hepatocyte growth factor (HGF), keratinocyte growth factor (KGF) and their receptors in human breast cells and tissues. Cell Mol. Biol. Res. 40:333–350.
S. Coleman-Kranick and J. Rosen (1994). Differential temporal and spatial gene expression of fibroblast growth factor family members during mouse mammary gland development. Mol. Endocrinol. 8:218–229.
W. Imagawa, G. Cunha, P. Young, and S. Nandi (1994). Keratinocyte growth factor and acidic fibroblast growth factor are mitogens for primary cultures of mammary epithelium. Biochem. Biophys. Res. Comm. 204:1165–1169.
V.K. Pedchenko and W.T. Imagawa (1998). Mammogenic hormones differentially modulate keratinocyte growth factor (KGF)-induced proliferation and KGF receptor expression in cultured mouse mammary gland epithleium. Endocrinology 139:2519–2526.
Y. Yang, E. Spitzer, D. Meyer, M. Sachs, C. Niemann, G. Harmann, K.M. Weidner, C. Birchneier, and W. Birchneier (1995). Sequential requirement of hepatocyte growth factor and neuregulin in morphogenesis and differentiation of the mammary gland. J. Cell Biol. 131:215–226.
F.E. Jones, D.J. Jerry, B.C. Guarino, G.C. Andrews, and D.F. Stern (1996). Heregulin induces in vivo proliferation and differentiation of mammary epithelium into secretory lobuloalveoli. Cell Growth Differ. 7:1031–1037.
D.J. Riese II, J.M. Van Raaiji, G.D. Plowman, G.C. Andrews, and D.F. Stern (1995). Cellular response to neuregulins is governed by complex interactions of the erbβ receptor family. Mol. Cell. Biol. 15:5770–5776.
S.Z. Haslam, L.J. Counterman, and K.A. Nummy (1992). EGF receptor regulation in normal mouse mammary glands. J. Cell Physiol. 152:553–557.
W. Imagawa, G.K. Bandyopadhyay, and S. Nandi (1990). Regulations of mammary epithelial cell growth in mice and rats. Endocrine Rev. 11:494–523.
R.B. Clarke, A. Howell, C.S. Patten, and E. Anderson (1997). Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 57:4987–4991.
J. Russo, X. Ao, C. Grill, and I.H. Russo (1998). Pattern of distribution of cells positive for estrogen receptor α and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res. Treat. (in press).
L. Hennighausen and G.W. Robinson (1998). Think globally, act, locally: The making of a mouse mammary gland. Genes Dev. 12:449–455.
P. Sicinski, J.L. Donaher, S.B. Parker, T. Li, A. Fazeli, H. Gardner, S.Z. Haslam, R.T. Bronson, S.J. Elledge, and R.A. Weinberg (1995). Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82:621–630.
S.M. Aronica, W.L. Krauss, and B.S. Katzenellenbogen (1994). Estrogen action via the cAMP signaling pathway: Stimulation of adenylate cyclase and CAMP regulated gene transcription. Proc. Natl. Acad. Sci. U.S.A. 91:8517–8521.
M. Beato, P. Herrlich, and G. Schultz (1995). Steroid hormone receptors: Many actors in search of a plot. Cell 83:851–857.
J.D. Graham, C. Yeates, R.L. Balleine, S.S. Harvey, J.S. Milliken, M. Bilous and C.L. Clarke (1995). Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 55:5063–5068.
D.P. McDonnell and M.E. Goldman (1994). RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J. Biol. Chem. 269: 11945–11949.
D. Chalbos and F. Galtier (1994). Differential effect of forms A and B of human progesterone receptor on estradiiol-dependent transcription. J. Biol. Chem. 269:23007–23012.
W.L. Krauss, K.E. Weis, and B.S. Katzenellenbogen (1995).Inhibitory cross-talk between steroid hormone receptors: Dif-ferential targeting of estrogen receptor in the repression of its transcriptional activity by agonist and antagonist occupied progesterone receptors. Mol. Cell Biol. 15: 1847–1857.
A. Migliaccio, D. Piccolo, G. Castoria, M. DiDomenico, A. Bilancio, M. Lombardi, W. Gong, M. Beato, and F. Auricchio (1998). Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 17:2008–2018.
Rights and permissions
About this article
Cite this article
Shyamala, G. Progesterone Signaling and Mammary Gland Morphogenesis. J Mammary Gland Biol Neoplasia 4, 89–104 (1999). https://doi.org/10.1023/A:1018760721173
Issue Date:
DOI: https://doi.org/10.1023/A:1018760721173