Abstract
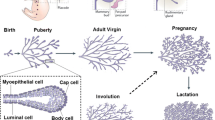
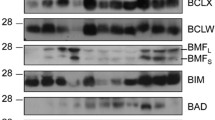
Ductal development in the pubertal mouse mammarygland is characterized by dramatic morphological changesin the epithelium driven by proliferation of cap andbody cells in the terminal endbuds. Recent experiments revealed a coincident and abundantapoptosis in the body cells of these structures. Thecells undergoing apoptosis are occasionally restrictedto defined regions within the terminal endbud. Localization adjacent to the presumptive luminasuggests that this process functions to sculpt thelumina of the subtending duct. Members of the Bcl-2family of apoptosis regulatory molecules; Bcl-2 and Bcl-x, appear to have some role in regulatingapoptosis in the terminal endbud. Other possible signalswhich could regulate this developmental process and amodel are presented.
Similar content being viewed by others
REFERENCES
A. Glucksmann (1951). Cell death in normal vertebrate ontogeny. Biol. Rev. 26:59–86.
J. W. Saunders (1966). Death in embryonic systems. Science 154:604–612.
M. D. Jacobson, M. Weil, and M. C. Raff (1997). Programmed cell death in animal development. Cell 88:347–354.
H. S. Coles, J. F. Burne, and M. C. Raff (1993). Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development 118:777–784.
C. W. Daniel and G. B. Silberstein (1985). In M. Neville and C. Daniel (eds.) The Mammary Gland Development Regulation and Function, Plenum pp. 3–36.
R. C. Humphreys, M. Krajewska, S. Krnacik, R. Jaeger, H. Weiher, S. Krajewski, J. C. Reed, and J. M. Rosen (1996). Apoptosis in the terminal endbud of the murine mammary gland: A mechanism of ductal morphogenesis. Development 122:4013–4022.
R. Dulbecco, M. Henahan, and B. Armstrong (1982). Cell types and morphogenesis in the mammary gland. Proc. Natl. Acad. Sci. U.S.A. 79:7346–7350.
R. Dulbecco, M. Unger, B. Armstrong, M. Bowman, and P. Syka (1983). Epithelial cell types and their evolution in the rat mammary gland determined by immunological markers. Proc. Natl. Acad. Sci. U.S.A. 80:1033–1037.
C. H. Knight and M. Peaker (1982). Development of the mammary gland. J. Reprod. Fertil. 65:521–536.
D. J. Pierce, M. D. Johnson, Y. Matsui, S. D. Robinson, L. I. Gold, A. F. Purchio, C. W. Daniel, B. L. Hogan, H. L. Moses (1993). Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev. 7:2308–2317.
G. B. Silberstein, H. K. Van, G. Shyamala, and C. W. Daniel (1994). Essential role of endogenous estrogen in directly stimulating mammary growth demonstrated by implants containing pure antiestrogens. Endocrinology 134:84–90.
G. B. Silberstein, P. Strickland, S. Coleman, and C. W. Daniel (1990). Epithelium-dependent extracellular matrix synthesis in transforming growth factor-beta 1–growth-inh ibited mouse mammary gland. J. Cell Biol. 110:2209–2219.
R. C. Humphreys, J. Lydon, B. W. O'Malley, and J. M. Rosen (1997). Mammary gland development is mediated by both stromal and epithelial progesterone receptors. Mol. Endocrinol. 11:801–811.
C. W. Daniel and S. D. Robinson (1992). Regulation of mammary growth and function by TGF-beta. Mol. Repro. Dev. 32:145–151.
C. W. Daniel, G. B. Silberstein, and P. Strickland (1987). Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Res. 47:6052–6057.
S. Coleman and C. W. Daniel (1990). Inhibition of mouse mammary ductal morphogenesis and down-regulation of the EGF receptor by epidermal growth factor. Dev. Biol. 137: 425–433.
W. Ruan, C. B. Newman, D. L. Kleinberg (1992). Intact and amino-terminally shortened forms of insulin-like growth factor I induce mammary gland differentiation and development. Proc. Natl. Acad. Sci. U.S.A. 89:10872–10876.
P. D. Walden, W. Ruan, M. Feldman, and D. L. Kleinberg (1998). Evidence that the mammary fat pad mediates the action of growth hormone in mammary gland development. Endocrinology 139:659–662.
Y. Gavrieli, Y. Sherman, and S. S. Ben (1992). Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119:493–501.
G. B. Silberstein, K. Van Horn, G. Shyamala, and C. W. Daniel (1996). Progesterone receptors in the mouse mammary duct. Cell Growth Differ. 7:945–952.
Z. Feng, A. Marti, B. Jehn, H. J. Altermatt, G. Chicaiza, and R. Jaggi (1995). Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. J. Cell Biol. 131:1095–1103.
D. L. Hadsell, N. M. Greenberg, J. M. Fligger, C. R. Baumrucker, and J. M. Rosen (1996). Targeted expression of des (1–3) human insulin-like growth factor I in transgenic mice influences mammary gland development and IGF-binding protein expression [see comments]. Endocrinology 137: 321–330.
S. Coleman, G. B. Silberstein, and C. W. Daniel (1988). Ductal morphogenesis in the mouse mammary gland: Evidence supporting a role for epidermal growth factor. Dev. Biol. 127: 304–315.
W. Imagawa, Y. Tomooka, S. Hamamoto, and S. Nandi (1985). Stimulation of mammary epithelial cell growth in vitro: Interaction of epidermal growth factor and mammogenic hormones. Endocrinology 116:1514–1524.
G. R. Merlo, F. Basolo, L. Fiore, L. Duboc, and N. E. Hynes (1995). p53–dependent and p53–independent activation of apoptosis in mammary. J. Cell Biol. 128:1185–1196.
T. M. Casey, H. Chen, K. Plaut, and J. F. Chiu (1996). Involution of mouse mammary glands during whole organ culture occurs. Cell. Biol. Int. 20:763–767.
G. B. Silberstein, K. C. Flanders, A. B. Roberts, and C. W. Daniel (1992). Regulation of mammary morphogenesis: Evidence for extracellular matrix-mediated inhibition of ductal budding by transforming growth factor-beta 1. Dev. Biol. 152:354–362.
E. A. Harrington, A. Fanidi, and G. I. Evan (1994). Oncogenes and cell death. [Review]. Curr. Opin. Gene. Dev. 4:120–129.
J. C. Reed (1994). Bcl-2 and the regulation of programmed cell death. [Review]. J. Cell Biol. 124:1–6.
S. J. Korsmeyer, J. R. Shutter, D. J. Veis, D. E. Merry, and Z. N. Oltvai (1993). Bcl-2/Bax: A rheostat that regulates an anti-oxidant pathway and cell death. [Review]. Sem. Cancer Biol. 4:327–332.
M. Li, J. Hu, K. Heermeier, L. Henninghausen, and P. Furth (1996). Apoptosis and remoldeling of mammary gland tissue during involution proceeds through p53–independent pathways. Cell Growth Differ. 7:13–20.
L. A. Donehower, M. Harvey, B. L. Slagle, M. J. McArthur, C. A. J. Montgomery, J. S. Butel, and A. Bradley (1992). Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumors. Nature 356:215–221.
G. B. Silberstein and C. W. Daniel (1982). Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev. Biol. 90:215–222.
P. J. Keely, J. E. Wu, and S. A. Santoro (1995). The spatial and temporal expression of the alpha 2 beta 1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation 59: 1–13.
Z. Zhang, K. Vuori, J. C. Reed, and E. Ruoslahti (1995). The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc. Natl. Acad. Sci. U.S.A. 92:6161–6165.
S. M. Frisch and H. Francis (1994). Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124: 619–626.
N. Boudreau, C. J. Sympson, Z. Werb, and M. J. Bissell (1995). Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267:891–893.
R. Khokha, D. C. Martin, and J. E. Fata (1995). Utilization of transgenic mice in the study of matrix degrading proteinases and their inhibitors. [Review]. Cancer Metastasis Rev. 14: 97–111.
J. P. Witty, J. H. Wright, and L. M. Matrisian (1995). Matrix metalloproteinases are expressed during ductal and alveolar. Mol. Biol. Cell 6:1287–1303.
E. Coucouvanis and G. R. Martin (1995). Signals for death and survival: A two-step mechanism for cavitation in the vertebrate embryo. Cell 83:279–287.
C. W. Daniel, P. Strickland, and Y. Friedmann (1995). Expression and functional role of E-and P-cadherins in mouse mammary ductal morphogenesis and growth. Dev. Biol. 169: 511–519.
J. Russo and I. H. Russo (1978). DNA labeling index and structure of the rat mammary gland as determinants of its susceptibility to carcinogenesis. J. Natl. Cancer Inst. 61: 1451–1459.
J. Russo, G. Wilgus, and I. H. Russo (1979). Susceptibility of the mammary gland to carcinogenesis: I Differentiation of the mammary gland as determinant of tumor incidence and type of lesion. Am. J. Path. 96:721–736.
J. Russo and I. H. Russo (1987). Biological and molecular bases of mammary carcinogenesis. [Review]. Lab. Invest. 57: 112–137.
K. T. Christov, R. C. Guzman, S. M. Swanson, G. Thordarson, F. Talamantes, and S. Nandi (1996). Cell proliferation and apoptosis during mammary carcinogenesis in pituitary isografted mice. Carcinogenesis 17:1741–1746.
G. I. Evan, L. Brown, M. Whyte, and E. Harrington (1995). Apoptosis and the cell cycle. [Review]. Curr. Opin. Cell Biol. 7:825–834.
Rights and permissions
About this article
Cite this article
Humphreys, R.C. Programmed Cell Death in the Terminal Endbud. J Mammary Gland Biol Neoplasia 4, 213–220 (1999). https://doi.org/10.1023/A:1018733426625
Issue Date:
DOI: https://doi.org/10.1023/A:1018733426625