Abstract
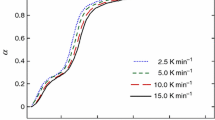
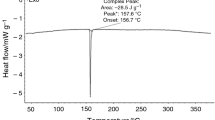
Hexa-ammonium tetraphosphate dihydrate, (NH4)6P4O13·2H2O (HATP), was prepared by the hydrolysis of sodium cyclo-tetraphosphate with sodium hydroxide solution, followed by ion-exchange with ammonium. Thermal decomposition in static air was first carried out dynamically, at a heating rate of 5 K min-1 as used in thermal analysis (thermogravimetric-differential thermal analysis), and also isothermally. To examine the effect of humidity on the thermal decomposition, HATP was heated isothermally in streams of dry and humid air. The products were characterized by X-ray diffraction analysis and high-performance liquid chromatography–flow injection analysis. At 100°C, HATP was decomposed to mono- and triphosphates and to 2 mol diphosphate, and this was accelerated by humidity. Further degradation of the triphosphate to mono- and diphosphates took place slowly. The 2 mol diphosphate also decomposed slowly to 4 mol monophosphate. At temperatures above 150°C, the form I of ammonium polyphosphate (I-APP) was produced. I-APP was further hydrolysed by humidity to shorter-chain phosphates, such as mono-, di- and triphosphates.
Similar content being viewed by others
References
T. D. FARR and J. W. WILLIARD, J. Chem. Eng. Data 17 (1972) 317.
E. KOBAYASHI, Phosphorus Lett. 5 (1988) 3.
M. N. NABIEV, M. T. SAIBOVA, I. A. BORUKHOV and N. A. PARPIEV, Zh. Neorg. Khim. 14 (1969) 2950.
D. BOHUSLAV, K. FRANTISEK and J. FRANTISEK, Sb. Vys. Sk. Chem.-Technol. Praze, Anorg. Chem. Technol. B15 (1972) 31.
L. G. BEREZKINA and S. I. BORISOVA, Zh. Prikl. Khim. (Leningrad) 57 (1984) 1372.
M. MACIEJEWSKI and R. RUDNICKI, Thermochim. Acta 113 (1987) 305.
A. TAKENAKA, O. TAKEO, N. MORIYAMA, I. MOTOOKA and H. NARIAI, Chem. Express 8 (1993) 213.
E. KOBAYASHI, Nippon Kagaku Zasshi 83 (1962) 132.
R. P. RILO, E. P. SAMOKHVALOV and I. M. KAGANSKII, Zh. Prikl. Khim. (Leningrad) 56 (1983) 879.
A. W. FRAZIER, J. P. SMITH and J. R. LEHR, J. Agr. Food Chem. 13 (1965) 317.
V. A. SOTNIKOVA-YUZHIK, M. M. PAVLYUCHENKO and E. A. PRODAN, Vestsi Akad. Navuk B. Ser. Khim. Navuk 5 (1975) 32.
E. A. PRODAN, L. I. PETROVSKAYA, M. M. PAVLYUCHENKO and N. A. AKULICH, ibid. 2 (1976) 11.
A. MENLIBAEV, D. Z. SERAZETDINOV, M. Kh. KIM and Zh. K. SHAIDARBEKOVA, Izv. Akad. Nauk Kaz. SSR, Ser. Khim. 27 (1977) 1.
L. I. PETROVSKAYA, E. A. PRODAN, N. G. RAFAL'sKII and N. V. KHARITONCHIK, Vestsi Akad. Navuk B SSR, Ser. Khim. Navuk 4 (1980) 44.
E. A. PRODAN and V. I. KORZHUEV, Zh. Neorg. Khim. 28 (1983) 24.
E. A. PRODAN, V. N. KORZHUEV and L. I. PETROVSKAYA, ibid. 29 (1984) 1679.
E. J. GRIFFITH, J. Inorg. Nucl. Chem. 26 (1964) 138.
T. D. FARR, J. W. WILLIARD and J. D. HATFIELD, J. Chem. Eng. Data 17 (1972) 313.
K. R. WAERSTAD and G. H. McCLELLAN, J. Appl. Crystallogr. 7 (1974) 404.
M. WATANABE, H. SUZUMORI, M. MAEDA and T. YAMADA, Bull. Chem. Soc. Jpn. 53 (1980) 2663.
M. WATANABE, M. MAEDA and T. YAMADA, ibid. 56 (1983) 3430.
M. WATANABE, H. SUZUMORI, M. MAEDA and T. YAMADA, Gypsum and Lime 178 (1982) 3.
A. TAKENAKA, I. MOTOOKA and H. NARIAI, Bull. Chem. Soc. Jpn. 60 (1987) 4299.
R. N. BELL, L. F. AUDRIETH and F. HILL, Ind. Eng. Chem. 44 (1952) 568.
R. V. COATES and G. D. WOODARD, J. Chem. Soc. (1964) 1780.
Y. BABA and M. TSUHAKO, Jasco Report 23 (1988) 7.
C. Y. SHEN, N. E. STAHLHEBER and D. R. DYROFF, J. Am. Chem. Soc. 91 (1969) 62.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takenaka, A., Fukuma, Y., Yasuda, A. et al. Preparation and thermal decomposition of hexa-ammonium tetraphosphate dihydrate(NH4)6P4O13·2H2O. Journal of Materials Science 32, 3049–3053 (1997). https://doi.org/10.1023/A:1018673928201
Issue Date:
DOI: https://doi.org/10.1023/A:1018673928201