Abstract
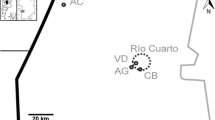
Blood from approximately 400 crucian carp, Carassius carassius, from 12 ponds in the Ukraine, was analysed by flow cytometry to assess the possible relationships between chronic contaminant exposure and variation in the cellular DNA content. The ponds were located 20--30 km from Chernobyl in areas that received 3.7 × 1010--3.7 × 1011 Bq 137Cs km-2 after the 1986 nuclear accident, as well as other radioactive and chemical contaminants. The fish populations consisted of both diploid and triploid individuals and the ploidy varied between ponds. Analysis of whole blood revealed aneuploid-like patterns in the DNA histograms of some fish, as well as hyperdiploid shoulders of the G0/G1 peak. The coefficient of variation (CV) of the G0/G1 peak has previously been employed to assess the variability in the DNA content of cells within individuals. The CV of individual fish varied between and within locations and very large CVs were found for some individuals. In some fish, DNA histograms showed a typical diploid or triploid cell population together with a smaller haploid population. Variations in the cellular DNA content similar to those reported here have been associated with exposure to radiation and other genotoxic agents in laboratory and field studies. However, the abnormalities we observed were not correlated with known contaminant distributions. While further work is needed, particuarly in areas with substantially higher levels of radioactivity, these results suggest that the Chernobyl accident may have long-term genetic consequences for wild organisms inhabiting contaminated areas
Similar content being viewed by others
References
Abramov, V.I., Fedorenko, O.M. and Shevchenko, V.A. (1992) Genetic consequences of radioactive contamination for populations of Arabidopsis. Sci. Total Environ. 112, 19–28.
Allen, S.K. and Wattendorf (1987) Triploid grass carp: status and management implications. Fisheries (Bethesda) 12, 20–4.
Anspaugh, L.R., Caitlin, R.J. and Goldman, M. (1988) The global impact of the Chernobyl reactor accident. Science 242, 1513–19.
Barlogie, B., Stass, S., Dixon, D., Keating, M., Cork, A., Trujillo, J.M., McCredie, K.B. and Freireich, E.J. (1987) DNA aneuploidy in adult acute leukemia cancer. Genet. Cytogenet. 28, 213–28.
Bickham, J.W., Hanks, B.G., Smolen, M.J., Lamb, T. and Gibbons, J.W. (1988) FLow cytometric analysis of the effects of low-level radiation exposure on natural populations of slider turtles (Pseudemys scripta). Arch. Environ. Contam. Toxicol. 17, 837–41.
Cherfas, N.B. (1966) Natural triploidy in the females of the unisexual variety of the silver crucian carp (C. auratus gibelio Bloch). Genetika (Moscow) 2(5), 16–24.
Custer, T.W., Bickham, J.W., Lyne T.B., Lewis, T., Ruedas, L.A., Custer, C.M. and Melancon, M.J. (1994) Flow cytometry for monitoring contaminant exposure in black-crowned night herons. Arch. Environ. Contam. Toxicol. 27, 176–9.
Dallas, C.E. (1993) Aftermath of the Chernobyl nuclear disaster: pharmaceutical needs in the republic of Belarus. Am. J. Pharmaceut. 57, 182–5.
Dallas, C.E. and Evans, D.E. (1991) Flow cytometry in toxicity analysis. Nature 345, 557–8.
Deaven, L.L. (1982) Application of flow cytometry to cytogenetic testing of environmental mutagens. In T.C. Hsu (ed.), Cytogenetic assays of environmental mutagens, pp. 325–51. Totowa, NJ: Allanheld, Osmun and Co. Pub., Inc.
Fan, Z. and Shen, J. (1990) Studies on the evolution of bisexual reproduction in crucian carp (Carassius auratus gibelio Block). Aquaculture 84, 235–44.
Fernö, M., Baldetorp, B., Ewers, S., Idvall, I., Olsson, H., Sigurdsson, H. and Killander, D. (1992) One or multiple samplings for flow cytometric DNA analyses in breast cancer — prognostic implications? Cytometry 13, 241–9.
Fertig, G. and Miltenburger, H. (1989) Flow-cytometric cell-cycle analysis of Chinese hamster cells following exposure to cytotoxicants. Mutat. Res. 215, 61–8.
Finstad, J., Fange, R. and Good, R.A. (1969) The development of the lymphoid system: immune response and radiation sensitivity in lower vertebrates. Adv. Exp. Med. Biol. 5, 21–31.
Fisher, S.K., Dallas, C.E., Jagoe, C.H., Smith, M.H., Brisbin, I.L., Jr and Chesser, R.K. (1994) Sources of error associated with sample collection and preparation of nucleated blood cells for flow cytometric analysis. Cell Biol. Toxicol. 10, 145–53.
Friedlander, M., Hedley, D.W. and Taylor, I.W. (1984) Clinical and biological significance of aneuploidy in human tumors. J. Clin. Pathol. 37, 961–74.
George, L.S., Dallas, C.E., Brisbin, I.L., Jr and Evans, D.L. (1991) Flow cytometric analysis of ducks accumulating 137Cs on a reactor reservoir. Ecotoxicol. Environ. Safety 21, 337–47.
Gervai, J., Marian, T., Krasznai, Z., Nagy, A. and Csanyi, V. (1980) Occurrence of aneuploidy in radiation gynogenesis of carp, Cyprinus carpio L. J. Fish Biol. 16, 435–9.
Gold, J.R., Karel, W.J. and Strand, M.R. (1980) Chromosome formulae of North American fishes. Prog. Fish Cult. 42, 10–33.
International Atomic Energy Agency (1991) The International Chernobyl Project. Surface Contamination Maps. Vienna: IAEA.
Jagoe, C.H., Chesser, R.K., Smith, M.H., Dallas, C.E. and Fisher, S.K. (1993) Distribution of radionuclides and other contaminants in ponds near Chernobyl, Ukraine. In Ecological Risk Assessment: Lessons Learned?, p. 465.
Kazakov, V.S., Demidchik, E.P. and Astakhova, L.N. (1992) Thyroid cancer after Chernobyl. Nature 359, 21–2.
Lamb, T., Bickham, J.W., Gibbons, J.W., Smolen, M.J. and McDowell, S. (1991) Genetic damage in a population of slider turtles (Trachemys scripta) inhabiting a radioactive reservoir. Arch. Environ. Contam. Toxicol. 20, 138–42.
Lamb, T., Bickham, J.W., Lyne, T.B. and Gibbons, J.W. (1995) The slider turtle as an environmental sentinel: multiple tissue assays using flow cytometric analysis. Ecotoxicology 4, 5–13.
Liang, J.C. and Brinkley, B.R. (1995) Chemical probes and possible targets for the induction of aneuploidy. Aneuploidy 36, 491–505.
McBee, K. and Bickham, J.W. (1988) Petrochemical-related DNA damage in wild rodents detected by flow cytometry. Bull. Environ. Contam. Toxicol. 40, 343–9.
McFadden, P.W., Clowry, L.J., J., Daehnert, K., Hause, L.L. and Koethe, S.M. (1990) Image analysis confirmation of DNA aneuploidy in flow cytometric DNA distributions having a wide coefficient of variation of the G 0/G 1 peak. Am. J. Clin. Pathol. 93, 637–42.
Medvedev, Z.A. (1990) The Legacy of Chernobyl. NY: W.W. Norton & Company.
Medvedev, Z.A. (1994) Chernobyl: eight years after. Trends Ecol. Evol. 9, 369–71.
Miller, G.D., Seeb, J.E., Bue, B.G. and Sharr, S. (1994) Saltwater exposure at fertilization induces ploidy alterations, including Mosaicism, and Salmonids. Can. J. Fish. Aquat. Sci. 51, 42–9.
Mourad, R. and Snell, V. (1987) Source term and radiological consequences of the Chernobyl accident. Trans. Am. Nuclear Soc. 54, 226–8.
Oshimura, M. and Barrett, J.C. (1986) Chemically induced aneuploidy in mammalian cells: mechanisms and biological significance in cancer. Environ. Mutagen. 8, 129–59.
Otto, F.J. and Oldiges, H. (1980) Flow cytogenetic studies in chromosomes and whole cells for the detection of clastogenic effects. Cytometry 1, 13–17.
Otto, F.J., Oldiges, H. and Jain, V.K. (1984) Flow cytometric measurement of cellular DNA content dispersion induced by mutagenic treatment. In W.G. Eisert and M.L. Mendelsohn (eds), Biological dosimetry, pp. 36–49. Springer-Verlag.
Pinkel, D., Gledhill, B.L., VanDilla, M.A., Lake, S. and Wyborek, A.J. (1983) Radiation-induced DNA content variability in mouse sperm. Radiat. Res. 95, 550–65.
Powers, D.A., Kress, T.S. and Jankowski, M.W. (1987) The Chernobyl source term. Nuclear Safety 28, 10–28.
Prisyazhiuk, A., Pjatak, O.A., Buzanov, V.A., Reeves, C.K. and Beral, V. (1991) Cancer in the Ukraine: post-Chernobyl. Lancet 338, 1334–5.
Refstie, T. (1981) Tetraploid rainbow trout produced by cytochalasin B. Aquaculture 25, 51–8.
Refstie, T., Vassvik, V. and Gjedrem, T. (1977) Induction of polyploidy in salmonids by cytochalasin B. Aquaculture 10, 65–74.
Shevchenko, V.A., Pomerantseva, M.D., Ramaiya, L.K., Chekhovich, A.V. and Testov, B.V. (1992) Genetic disorders in mice exposed to radiation in the vicinity of the Chernobyl nuclear power station. Sci. Total Environ. 112, 45–56.
Sich, A.R. (1994) Chernobyl accident management actions. Nuclear Safety 35, 1–24.
Smith, L.T. and Lemoine, H.L. (1979) Colchicine-induced polyploidy in brook trout. Prog. Fish Cult. 41, 86–8.
Sokal, R.R. and Rohlf, F.J. (1969) Biometry. San Franscisco: W.H. Freeman.
Theodorakis, C.W., D'Surney, S.J., Bickham, J.W., Lyne, T.B., Bradley, B.P., Hawkins, W.E., Farkas, W.L., McCarthy, J.F. and Shugart, L.R. (1992) Sequential expression of biomarkers in bluegill sunfish exposed to contaminated sediment. Ecotoxicology 1, 45–73.
Thilly, W.G. and Call, K.M. (1986) Genetic toxicology. In C.D. Klaassen, M.O. Amdur and J. Doull (eds), Casarett and Doull's toxicology, pp. 174–94. NY: Macmillan Publishing Co.
Tiersch, T.R. and Wachtel, S.S. (1993) Sources of error in screening by flow cytometry for the effects of environmental mutagens. Environ. Toxicol. Chem. 12, 37–42.
Van Dilla, M., Pinkel, D., Gledhill, B., Lake, S., Watchmaker, G. and Wyborek, A. (1980) FCM of mammalian sperm: progress report. In O.D. Laerum, T. Lindmo and E. Thorud (eds), Flow cytometry IV, pp. 279–83. Norway: Universitatsforlaget.
Vindelov, L.L., Christensen, I.J. and Nissen, N.I. (1982) A detergent-trypsin method for the separation of nuclei for flow cytometric DNA analysis. Cytometry 3, 323–7.
Watson, L.J., Shechmeister, I.L. and Jackson, L.L. (1962) The hematology of goldfish, Carassius auratus. Cytologia 28, 118–30.
Zainullin, V.G., Shevchenko, V.A., Mjasnjankina, E.N., Generalova, M.V. and Rakin, A.O. (1992) The mutation frequency of Drosophila melanogaster populations living under conditions of increased background radiation due to the Chernobyl accident. Sci. Total Environ. 112, 37–44.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lingenfelser, S., Dallas, C., Jagoe, C. et al. Variation in blood cell DNA in Carassius carassius from ponds near Chernobyl, Ukraine. Ecotoxicology 6, 187–203 (1997). https://doi.org/10.1023/A:1018622710184
Issue Date:
DOI: https://doi.org/10.1023/A:1018622710184