Abstract
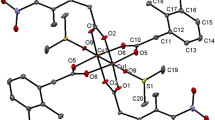
The reaction of H2CO and H2NCH2CH2OH with the nickel(II) complex of 1,9-diamino-3,7-diazanonane (2,3,2-tet) in the presence of Et3N gives the nickel(II) complex of the macrocycle 3-hydroxyethyl-1,3,5,8,12-pentaazacyclotetradecane (L), which can be readily isolated as the perchlorate salt. The reaction of KCNS with the perchlorate salt in aqueous solution gives [NiL(NCS)2] and the crystal structure of this complex has been determined. The complex is octahedral and trans with the two N-bonded thiocyanates in the axial sites with Ni-NCS bond lengths of 2.106 and 2.145AÅ. The equatorial sites are occupied by N2, N5, N8 and N12 with Ni-N bond distances of 2.053 to 2.076AÅ, which are typical for octahedral nickel(II) complexes. The ligand has a trans III configuration of the sec-NH centres, leading to chair six-membered rings and gauche five-membered rings. The hydroxyethyl group on N3 is axial. There is no evidence for hydrogen-bonding interactions involving the hydroxyethyl group in the crystal lattice.
Similar content being viewed by others
References
R. W. Hay, J. M. Armstrong and M. M. Hassan, Transition Met. Chem., 17, 270 (1992).
See, for example, L. Fabbrizzi, M. Lichelli, A. Poggi, O. Vassalli, L. Ungaretti and N. Sardone, Inorg. Chim. Acta, 246, 379 (1996); P. Comba, N. F. Curtis, G. A. Lawrance, A. M. Sargeson, B. W. Skelton and A. H. White, Inorg. Chem., 25, 4260 (1986); M. P. Suh and S-G. Kang, Inorg. Chem., 27, 2544 (1988); L. Xin, N. F. Curtis and D. C. Weatherburn, Transition Met. Chem., 17, 147 (1992); R. W. Hay and I. Fraser, Transition Met. Chem., 16, 9 (1991).
L. Ballester, M. C. Barral, A. Gutierrez, A. Monge, M. F. Perpinan, C. Ruiz-Valero and A. E. Sanchez-Pelaez, Inorg. Chem., 33, 2142 (1994).
See, for example, C. I. Smith, J. A. Crayston and R. W. Hay, J. Chem. Soc., Dalton Trans., 3267 (1993).
A. Altomare, M. C. Burla, M. Camalli, M. Cascarano, C. Giacovazzo, A. Guagliardi and G. Polidori, J. Appl. Crystallogr., 26, 343 (1993).
P. T. Beurskens, G. Admiraal, G. Beurskens, W. P. Bosman, R. de Gelder, R. Israel and J. M. M. Smits. The DIRDIF-94 program system. Technical report of the Crystallography Laboratory, University of Nijmegen, The Netherlands.
The function minimised was Σw( Fo–Fc )2. All calculations were performed using the teXsan crystallographic software package of the Molecular Structure Corporation, Houston (1985 and 1992).
L. Fabbrizzi, A. M. Lanfredi, P. Pelavicini, A. Taglietti and F. Ugozzoli, J. Chem. Soc., Dalton Trans., 3263 (1991).
R. W. Hay, A. Danby, P. Lightfoot and Y. D. Lampeka, Polyhedron, (in press).
R. W. Hay, J. A. Crayston, T. J. Cromie, P. Lightfoot and D. Chanaka L. de Alwis, Polyhedron, (in press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hay, R.W., Cromie, T.J. & Lightfoot, P. The synthesis and crystal structure of [NiL(NCS)2] (L=3-hydroxyethyl-1,3,5,8,12-pentaazacyclotetradecane). Transition Metal Chemistry 22, 510–512 (1997). https://doi.org/10.1023/A:1018575632645
Issue Date:
DOI: https://doi.org/10.1023/A:1018575632645