Abstract
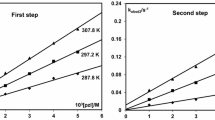
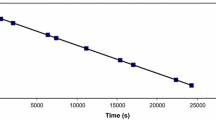
The reaction of dichloroarylazopyridinepalladium(II) [Pd(aap)Cl2, aap=4-R′C6H4N-N-2-C5H4N; R′= H (1), Me (2), Cl (3)] with pyridine bases [RPy: R-H (a), 2-Me(b), 4-Me(c), 4-Cl(d), 2-Ph(e), 2-PhCH2(f)] has been studied spectrophotometrically in MeCN at 400nm. The products (4) have been isolated and characterized as trans-Pd(RPy)2Cl2. The kinetics of the nucleophilic substitution have been examined under pseudo-first-order conditions with respect to base at 298K and follow the rate law, Rate=k[RPy]2 [Pd(aap)Cl2]. The rate data supports a nucleophilic association path. External addition of Cl− (LiCl) suppresses the rate, which follows the order: k(3)> k(1)>k(2), where k values are linearly related to Hammet σ constants. 2-Substitution in the pyridine base remarkably reduces the rate compared with 4-substitution and is attributed to a steric effect that destabilizes the transition state. The rate decreases with increasing steric crowding at the ortho-position and follows the order: (e)>(f)>(b). The 4-substituted pyridines control the rate via the inductive effect and follow the order: (d)>(a)>(c).
Similar content being viewed by others
References
F. Basolo and R. G. Pearson, Mechanism of Inorganic Reactions: A Study of Metal Complexes in Solution, 2nd Edit., Wiley, New York, 1967; (b) E. C. Constable, Polyhedron, 2, 551 (1983); (c) R. G. Wilkins, Kinetics and Mechanism of Reactions of Transition Metal Complexes, 2nd Edit., VCH, Weinheim 1991; (d) R. Romeo, G. Arena, L. M. Scolaro, M. R. Plutino, Inorg. Chim. Acta, 240, 81 (1995); (e) M. Krumn, I. Mutikainen and B. Lippert, Inorg. Chem., 30, 884 (1991).
G. Schroder, B. Lippert, M. Sabat, C. J. L. Lock, K. Faggiani, B. Song and H. Sigel, J. Chem. Soc., Dalton Trans., 3767 (1995); (b) R. B. Martin in S. Lippard (Ed.), Platinum, Gold and Other Metal Chemotherapeutic Agents, Am. Chem. Soc. Rep., 1983, Vol. 209, p. 231.
S. A. Kane and S. J. Lippard, Biochemistry, 35, 2180 (1996); (b) K. J. Barnham, M. I. Djuran, P. S. Mudoch, J. D. Randford and P. J. Sadler, Inorg. Chem., 35, 1065 (1996).
G. Annibale, L. Cattalini, A. A. El-Awady and G. Natile, J. Chem. Soc., Dalton Trans., 802 (1974); (b) J. S. Coe, J. R. Lyons and M. D. Hussain, J. Chem. Soc. (A), 90 (1970).
P. Haake and R. M. Pfeifer, J. Am. Chem. Soc., 92, 4991, 5243 (1970); (b) J. J. MacDougall, J. H. Nelson and F. Mathey, Inorg. Chem., 21, 2145 (1982); (c) J. J. MacDougall, J. H. Nelson and F. Mathey, Inorg. Chem., 19, 709, 1400 (1980).
C. K. Pal. S. Chattopadhyay, C. Sinha, D. Bandyopadhyay and A. Chakravorty, Polyhedron, 18, 999 (1994).
S. Goswami, A. R. Chakravorty and A. Chakravorty, Inorg. Chem., 20, 2245 (1981).
R. J. H. Clark and C. S. Williams, Inorg. Chem., 4, 350 (1965).
Y. Wakatsuki, H. Yamazaki, P. A. Grutsch, M. Santhanam and C. Kutal, J. Am. Chem. Soc., 107, 8153 (1985).
R. W. Hay, A. K. Basak, J. Chem. Soc., Dalton Trans., 1819 (1982); (b) F. L. Wimmer and S. Wimmer, Inorg. Chim. Acta, 149, 1 (1988); (c) K. Inagaki, A. Alinkar, A. Nagai, Y. Kidani and J. Reedjk, Inorg. Chem., 29, 2183 (1990); (d) G. Alibrandi, M. Cusumano, A. Giannello and D. Minniti, J. Chem. Soc., Dalton Trans., 375 (1989); (e) S. Wimmer, P. Castan, F. L. Wimmer and N. P. Johnson, Inorg. Chim. Acta, 142, 13 (1988).
B. K. Ghosh and A. Chakravorty, Coord. Chem. Rev., 95, 239 (1989).
D. G. Coper and J. Powell, J. Am. Chem. Soc., 95, 1102 (1973).
L. Canovese, M. L. Tobe and L. Cattalini, J. Chem. Soc., Dalton Trans., 27 (1985); (b) G. Natile, L. Maresea and L. Cattalini, J. Chem. Soc., Dalton Trans., 651 (1977).
C. Sinha, Transition Met. Chem., 19, 41 (1994).
C. Sinha, Ph.D. Thesis, Jadavpur University, India, 1990.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roy, R., Misra, T.K., Sinha, C. et al. Kinetics and mechanism of nucleophilic substitution of dichloroarylazopyridinepalladium(II) by pyridine bases. Transition Metal Chemistry 22, 453–458 (1997). https://doi.org/10.1023/A:1018550927193
Issue Date:
DOI: https://doi.org/10.1023/A:1018550927193