Abstract
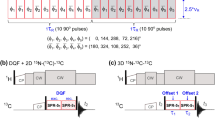
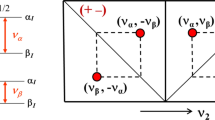
A modified HNHB experiment is presented that allows thedetermination of J(NH) coupling constants directly from the ratio ofcross-peak to diagonal-peak intensities. The experiment was applied to thephotoactive yellow protein (PYP) and yielded the magnitude of 1173J(NHβ) coupling constants. In addition, 293J(NHα(i−1)) coupling constantscould be measured, providing information about the backbone angle ψ.These data, in conjunction with the magnitudes of the3J(HNHα) coupling constantsobtained from the HNHA spectrum, effectively discriminate the twopossibilities for the stereospecific assignment of theHα resonances in glycine residues. For all eight glycineresidues in PYP that were not subject to conformational averaging and hadnon-degenerate Hα resonance frequencies, the J-couplingdata, together with limited NOE data, yielded the stereospecific assignmentof the Hα resonances for these residues. In addition,reliable and precise φ,ψ dihedral constraints were also derived forthese residues from the J-coupling data.
Similar content being viewed by others
References
Archer, S.J., Ikura, M., Torchia, D.A. and Bax, A. (1991) J. Magn. Reson., 95, 636–641.
Bax, A., Vuister, G.W., Grzesiek, S., Delaglio, F., Wang, A.C., Tschudin, R. and Zhu, G. (1994) Methods Enzymol., 239, 79–105.
Bystrov, V.F. (1976) Prog. NMR Spectrosc., 10, 41–81.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277–293.
Emerson, S.D. and Montelione, G.T. (1992) J. Magn. Reson., 99, 413–420.
Gemmecker, G. and Fesik, S.W. (1991) J. Magn. Reson., 95, 208–213.
Griesinger, C., Sørensen, O.W. and Ernst, R.R. (1986) J. Chem. Phys., 85, 6837–6843.
Griesinger, C. and Eggenberger, U. (1992) J. Magn. Reson., 97, 426–434.
Karimi-Nejad, Y., Schmidt, J.M., Rüterjans, H., Schwalbe, H. and Griesinger, C. (1994) Biochemistry, 33, 5481–5492.
Kleywegt, G.J., Vuister, G.W., Padilla, A., Knechtel, R.M., Boelens, R. and Kaptein, R. (1993) J. Magn. Reson., 102, 166–176.
Madsen, J.C., Sørensen, O.W., Sørensen, P. and Poulsen, F.M. (1993) J. Biomol. NMR, 3, 239–244.
Montelione, G.T., Winkler, M.E., Rauenbuehler, P. and Wagner, G. (1989) J. Magn. Reson., 82, 198–204.
Pardi, A., Billeter, M. and Wüthrich, K. (1984) J. Mol. Biol., 180, 741–751.
Seip, S., Balbach, J. and Kessler, H. (1994) J. Magn. Reson., B104, 172–179.
Shaka, A.J., Keeler, J., Frenkiel, T. and Freeman, R. (1983) J. Magn. Reson., 52, 335–338.
Sklenar, V., Piotto, M., Leppik, R. and Saudek, V. (1993) J. Magn. Reson., A102, 241–245.
Sørensen, O.W., Eich, G.W., Levitt, M.H., Bodenhausen, G. and Ernst, R.R. (1983) Prog. NMR Spectrosc., 16, 163–192.
Tjandra, N., Grzesiek, S. and Bax, A. (1996) J. Am. Chem. Soc., 118, 6264–6272.
Tolman, J.R., Flanagan, J.M., Kennedy, M.A. and Prestegard, J.H. (1995) Proc. Natl. Acad. Sci. USA, 92, 9279–9283.
Vuister, G.W. and Bax, A. (1993a) J. Am. Chem. Soc., 115, 7772–7777.
Vuister, G.W. and Bax, A. (1993b) J. Magn. Reson., B102, 228–231.
Vuister, G.W., Yamazaki, T., Torchia, D.A. and Bax, A. (1993) J. Biomol. NMR, 3, 297–306.
Wang, A.C. and Bax, A. (1995) J. Am. Chem. Soc., 117, 1810–1813.
Wang, A.C. and Bax, A. (1996) J. Am. Chem. Soc., 118, 2483–2494.
Weisemann, R., Rüterjans, H., Schwalbe, H., Schleucher, J., Bermel, W. and Griesinger, C. (1994) J. Biomol. NMR, 4, 231–240.
Wider, G., Neri, D., Otting, G. and Wüthrich, K. (1989) J. Magn. Reson., 85, 426–431.
Xu, R.X., Olejniczak, E.T. and Fesik, S.W. (1992) FEBS Lett., 305, 137–143.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Düx, P., Whitehead, B., Boelens, R. et al. Measurement of 15N-1H coupling constants in uniformly 15N-labeled proteins: Application to the photoactive yellow protein. J Biomol NMR 10, 301–306 (1997). https://doi.org/10.1023/A:1018393225804
Issue Date:
DOI: https://doi.org/10.1023/A:1018393225804