Abstract
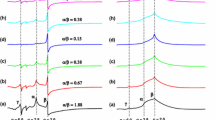
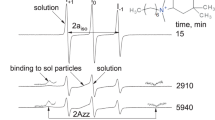
A spin probe TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidinyl-1-oxy) was dissolved in a tetraethyl orthosilicate sol-gel reaction system and measured by electron spin resonance spectroscopy at 295 K. The nitrogen hyperfine coupling constant was from 1.64–1.66 mT in the sol-gel solutions. The values were sensitive to the ethanol-to-water ratio of the solutions. The hyperfine coupling constant in the xerogels was 1.70 mT, which was almost the same as that in water, indicating that the probe molecules were trapped in silica pores with water adsorbed on the silica surfaces. The motion of TEMPOL in the xerogels was considerably slower than in the sol-gel solutions. The local viscosity estimated was from 70–90 cP. The ESR spectra of TEMPOL were altered during the sol-gel process, indicating that adsorbed water on the silica surfaces has an important role for trapping organic molecules in sol-gel glasses.
Similar content being viewed by others
References
J.I. Zink and B.S. Dunn, J. Ceram. Soc. Jpn. 99, 878 (1990).
D. Avnir, S. Braun, and M. Ottolenghi, in Supramolecular Architecture: Synthetic Control in Thin Films and Solids, edited by T. Bein (ACS Symposium Series No. 499, American Chemical Society, Washington, DC, 1992), p. 384.
D. Avnir, Acc. Chem. Res. 28, 328 (1995).
S. Sakka, J. Sol-Gel Sci. Tech. 3, 69 (1994).
(a) U. Narang, R. Wang, P.N. Prasad, and F.V. Bright, J. Phys. Chem. 98, 17 (1994). (b) U. Narang, J.D. Jordan, F.V. Bright, and P.N. Prasad, J. Phys. Chem. 98, 8101 (1994).
(a) S. Schreier, C.F. Polnaszek, and I.C.P. Smith, Biochimica et Biophysica Acta 515, 395 (1978). (b) J. Martinie, J. Michon, and A. Rassat, J. Am. Chem. Soc. 97, 1818 (1975). (c) B.R. Knauer and J.J Napier, J. Am. Chem. Soc. 98, 4395 (1976). (d) M.F. Ottaviani, P. Baglioni, and G. Martini, J. Phys. Chem. 87, 3146 (1983).
F. Mazzoleni, M.F. Ottaviani, M. Romanelli, and G. Martini, J. Phys. Chem. 92, 1953 (1988).
M. Romanelli, M.F. Ottaviani, G. Martini, and L. Kevan, J. Phys. Chem. 93, 317 (1989).
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, and T. Siemieniewska, Pure & Appl. Chem. 57, 603 (1985).
C.J. Brinker, K.D. Keefer, D.W. Schaefer, and C.S. Ashley, J. Non-Cryst. Solids 48, 47 (1982).
(a) K. Matsui and T. Nakazawa, Bull. Chem. Soc. Jpn. 63, 11 (1990). (b) K. Matsui, T. Nakazawa, and H. Morisaki, J. Phys. Chem. 95, 976 (1991). (c) K. Matsui, Langmuir 8, 673 (1992). (d) K. Matsui, M. Tominaga, Y. Arai, H. Satoh, and M. Kyoto, J. Non-Cryst. Solids 169, 295 (1994).
A. Shames, O. Lev, Y. Berkovich, and B. Iosefzon-Kuyavskaya, J. Sol-Gel Sci. and Tech. 2, 255 (1994).
K.C. Waterman, N.J. Turro, P. Chandar, and P. Somasundaran, J. Phys. Chem. 90, 6828 (1986).
S. Sakka and K. Kamiya, J. Non-Cryst. Solids 48, 31 (1982).
V.R. Kaufman and D. Avnir, Langmuir 2, 717 (1986).
F. Nishida, J.M. McKiernan, B. Dunn, J.I. Zink, C.J. Brinker, and A.J. Hurd, J. Am. Ceram. Soc. 78, 1640 (1995).
G. Martini, M.F. Ottaviani, and M. Romanelli, J. Colloid Interface Sci. 94, 105 (1983).
G. Martini, M.F. Ottaviani, M. Romanelli, and L. Kevan, Colloids and Surfaces 41, 149 (1989).
L.L. Hench and J.K. West, Chem. Rev. 90, 33 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsui, K., Kaneko, T., Yaginuma, Y. et al. ESR Study of a Nitroxide Radical in Sol-Gel Glasses. Journal of Sol-Gel Science and Technology 9, 273–277 (1997). https://doi.org/10.1023/A:1018359311661
Issue Date:
DOI: https://doi.org/10.1023/A:1018359311661