Abstract
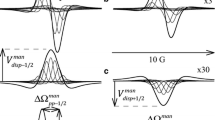
Tritium NMR spectroscopy has been used to examine the complexformed by [4-3H]benzenesulfon-amide and human carbonicanhydrase I. The results show that in solution the inhibitor forms a 1:1complex with the enzyme. A 100-spin computational model of the system,constructed with reference to crystallographic results, was used tointerpret tritium relaxation behavior and 3H{1H}NOEs. The analysis shows that the rate of dissociation of theenzyme–sulfonamide complex is 0.35 s−1 and thatthe aromatic ring of the inhibitor undergoes rapid rotation while complexed.
Similar content being viewed by others
References
Blackburn, G.M., Mann, B.E., Taylor, B.F. and Worrall, A.F. (1985) Eur. J. Biochem., 153, 553–558.
Boriack, P.A., Christianson, D.W., Kingery-Wood, J. and Whitesides, G.M. (1995) J. Med. Chem., 38, 2286–2291.
Chakravarty, S. and Kannan, K.K. (1994) J. Mol. Biol., 243, 298–309.
Chen, R.F. and Kernohan, J.C. (1967) J. Biol. Chem., 242, 5813–5823.
Creighton, T.E. (1984) Proteins, Freeman, New York, NY, U.S.A., p. 268.
Dodgson, S.J., Tashian, R.E., Gros, G. and Carter, N.D. (Eds.) (1991) The Carbonic Anhydrases, Plenum, New York, NY, U.S.A.
Doub, L. and Vandenbelt, J.M. (1947) J. Am. Chem. Soc., 69, 2714–2723.
Dugad, L.B. and Gerig, J.T. (1988) Biochemistry, 27, 4310–4316.
Dugad, L.B., Cooley, C.R. and Gerig, J.T. (1989) Biochemistry, 28, 3955–3960.
Feitl, M.E. and Krupin, T. (1991) In The Carbonic Anhydrases(Eds., Dodgson, S.J., Tashian, R.E., Gros, G. and Carter, N.D.), Plenum, New York, NY, U.S.A.
Gehring, K., Williams, P.G., Pelton, J.G., Morimoto, H. and Wemmer, D.E. (1991) Biochemistry, 30, 5524–5531.
Gerig, J.T. and Moses, J.M. (1987) J. Chem. Soc., Chem. Commun., 482–484.
Hansch, C., McClarin, J., Klein, T. and Langridge, R. (1985) Mol. Pharmacol., 27, 493–498.
Hansch, C. and Klein, T.E. (1986) Acc. Chem. Res., 19, 392–400.
Highsmith, S., Kubinec, M., Jaiswal, D.K., Morimoto, H., Williams, P.G. and Wemmer, D.E. (1993) J. Biomol. NMR, 3, 325–334.
Hunt, C.A., Mallorga, P.J., Michelson, S.R., Schwam, H., Sondey, J.M., Smith, R.L., Sugrue, M.F. and Shepard, K.L. (1994) J. Med. Chem., 37, 240–247.
Kanamori, K. and Roberts, J.D. (1983) Biochemistry, 22, 2658–2664.
Kannan, K.K., Petef, M., Fridborg, K., Cid-Dresdner, H. and Lovgren, S. (1977) FEBS Lett., 73, 115–119.
Kask, P., Piksarv, P., Mets, U., Pooga, M. and Lippmaa, E. (1987) Eur. Biophys. J., 14, 257–261.
King, R.W. and Burgen, A.S.V. (1976) Proc. R. Soc. London, B193, 107–125.
Kubinec, M.G., Culf, A.S., Cho, H., Lee, D.C., Burkham, J., Morimoto, H., Williams, P.G. and Wemmer, D.E. (1996) J. Biomol. NMR, 7, 236–246.
Kumar, V. and Kannan, K.K. (1994) J. Mol. Biol., 241, 226–232.
Liljas, A., Hakansson, K., Jonsson, B.H. and Xue, Y. (1994) Eur. J. Biochem., 219, 1–10.
Lindskog, S., Henderson, L.E., Kannan, K.K., Liljas, A., Nyman, P.O. and Strandberg, B. (1970) In The Enzymes, Vol. 5, 3rd ed. (Ed., Boyer, P.D.), Academic Press, New York, NY, U.S.A., pp. 587–665.
London, R.E. (1980) Magn. Reson. Biol., 1, 1–69.
Maren, T.H. (1967) Physiol. Rev., 47, 597–766.
Maren, T.H. and Sanyal, G. (1983) Annu. Rev. Pharmacol. Toxicol., {vn23}, 439–459.
Maren, T.H. (1987) Drug Dev. Res., 10, 255–276.
Maren, T.H. (1992) Mol. Pharmacol., 41, 419–426.
Menziani, M.C., de Benedetti, P.G., Gago, F. and Richards, W.G. (1989) J. Med. Chem., 32, 951–956.
Mushak, P. and Coleman, J.E. (1972) J. Biol. Chem., 247, 373–380.
Neuhaus, D. and Williamson, M.P. (1989) The Nuclear Overhauser Effect in Structural and Conformational Analysis, VCH, New York, NY, U.S.A.
Newmark, R.D., Un, S., Williams, P.G., Carson, P.J., Morimoto, M. and Klein, M.P. (1990) Proc. Natl. Acad. Sci. USA, 87, 583–587.
O’Connell, T.M., Gerig, J.T. and Williams, P.G. (1993) J. Am. Chem. Soc., 115, 3048–3055.
Olejniczak, E.T. (1989) J. Magn. Reson., 81, 392–394.
Shupe, J. (1942) J. Assoc. Agric. Chemists, 25, 227–232.
Sly, W.S. and Hu, P.Y. (1995) Annu. Rev. Biochem., 64, 375–401.
Supuran, C.T., Nicolae, A. and Popescu, A. (1996) Eur. J. Med. Chem., 31, 431–438.
Sylvia, L.A. and Gerig, J.T. (1995) Biochim. Biophys. Acta, 1251, 225–232.
Tropp, J. (1980) J. Chem. Phys., 72, 6035–6043.
Vedani, A. and Meyer Jr., E.F. (1984) J. Pharm. Sci., 73, 352–358.
Vedani, A., Huhta, D.W. and Jacober, S.P. (1989) J. Am. Chem. Soc., {vn111}, 4075–4081.
Yguerabide, J., Epstein, H.F. and Stryer, L. (1970) J. Mol. Biol., 51, 573–590.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Culf, A., Gerig, J. & Williams, P. Tritium NMR studies of the human carbonic anhydrase I–benzenesulfonamide complex. J Biomol NMR 10, 293–299 (1997). https://doi.org/10.1023/A:1018358101811
Issue Date:
DOI: https://doi.org/10.1023/A:1018358101811