Abstract
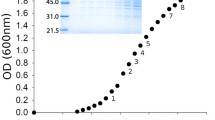
Staphylokinase, a profibrinolytic bacterial protein, was cloned into Escherichia coli, following the amplification of its gene via PCR. The amplificated gene was inserted in a pKK223-3 plasmid vector. The recombinant protein (STAR), expressed from a tac promoter, was obtained in the periplasmic space when IPTG was added to the culture medium. Both the concentration of the inducer as well as the growth phase of recombinant cells at which it was added affected the final yield of periplasmic STAR. The protein was purified by a one-step procedure in an acylated-plasminogen Sepharose coupled column.
Similar content being viewed by others
References
Bradford, M.M. (1976). Anal. Biochem. 72, 248–254.
Behnke, D. and Gerlach, D. (1987). Mol. Gen. Genet. 210, 528–534.
Collen, D., Silence, K., Demarsin, E., De Mol, M. and Lijnen, H.R. (1992). Fibrinolysis. 6, 203–213.
Collen, D., Van Hoef, B., Schlott, B., Hartmann, M., Guhrs, K-H. and Lijnen, H.R. (1993). Eur. J. Biochem. 216, 307–314.
Dagert, M. and Ehrlich, S.D. (1979). Gene. 6, 23–25.
Deutsch, D.G. and Mertz, E.T. (1970). Science. 170, 1095–1096.
Haqqi, T. (1992). Nuc. Acids. Res. 20, 6247.
Laemmli, U.K. (1970). Nature. 227, 680–685.
Lijnen, H.R., Van Hoef, B., De Cock, F., Okada, K., Ueshima, S., Matsuo, O. and Collen, D. (1991). J. Biol. Chem. 266, 11826–11832.
Lijnen, H.R., Van Hoef, B., Matsuo, O. and Collen, D. (1992). Biochim. Biophys. Acta. 1118, 144–148.
Malke, H. and Ferreti, J. (1984). Proc. Natl. Acad. Sci. USA. 81, 3557–3561.
Neu, H.C. and Heppel, I.A. (1965). J. Biol. Chem. 240, 3685–3692.
Sakai, M., Watanuki, M. and Matsuo, O. (1989). Biochem. Biophys. Res. Comm. 162, 830–837.
Sakamoto, S., Terada, Y., Iijima, M., Matsuzawa, H. and Ohta, T. (1994). Appl. Microbiol. Biotechnol. 42, 569–574.
Sako, T., Sawaki, S., Sakurai, T., Ito, S., Yoshizawa, Y. and Kondo, I. (1983). Mol. Gen. Genet. 190: 271–277.
Shimizu, N, Iijima, S. and Kobayashi, T. (1992). J. Ferment. Bioeng. 73, 163–168.
Silence, K., Collen, D. and Lijnen, H.R. (1993). J. Biol. Chem. 268, 9811–9816.
Vanderschueren, S., Barrios, L., Kerdsinchai, P., Van der Heuvel, P., Hermans, L., Vrolix, M., De Man, F., Benit, E., Muyldermans, L., Collen, D. and Van de Werf, F. (1995). Circulation. 92, 2044–2049.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yarzabal, A., Bastidas, M., Avilan, L. et al. Induction conditions for maximizing recombinant staphylokinase expression in Escherichia coli. Biotechnology Letters 19, 633–637 (1997). https://doi.org/10.1023/A:1018330613369
Issue Date:
DOI: https://doi.org/10.1023/A:1018330613369